Recently, an essential and universal receptor of Enterovirus B (EV-B) was uncovered, and the virus entry mechanism was explained by the joint team of George F. Gao’s lab from Institute of Microbiology, Chinese Academy of Sciences (CAS), Wensheng Wei’s lab from Beijing University, and Zhengde Xie’s lab from Beijing Children’s Hospital, Capital Medical University. The article entitled “Human neonatal Fc receptor is the cellular uncoating receptor for Enterovirus B” was published in Cell on May 16, 2019.
The Enterovirus B (EV-B) group consists of echovirus, coxsackievirus B (CV-B), coxsackievirus A9 (CV-A9), and other newly-identified enteroviruses. EV-B infections cause viral encephalitis (VE), viral meningitis (VM), and viral meningo-encephalitis (VME), leading to substantial morbidity and mortality in children. They are also the causative agents of acute flaccid paralysis (AFP), nonspecific rashes, pneumonitis, hepatitis, coagulopathy, and hand-foot-and-mouth disease (HFMD). In recent years, severe outbreaks of echovirus infections have been documented in America, Europe, and Asia. In China, surveillance of children with VE and VM in several provinces revealed that EV-B, especially echovirus, is the dominant serotype in the cerebrospinal fluid of patients. Thus far, there is no approved drug or vaccine specifically against EV-B infection, and no animal model for drug development. Thus, discovery of their potential receptor(s) and entry mechanism will paves the way for vaccine and drug development, and disease control.
Through CRISPR-Cas9 library screening, the team found that human neonatal Fc receptor (FcRn) is a universal and essential receptor for major EV-B such as echoviruses. As an immune factor, FcRn is hijacked by the virus for its entry. By obtaining multiple cryo-electron microscopy structures at different stages of virus entry at atomic or near-atomic resolution, the team deciphered the underlying mechanisms of enterovirus attachment and uncoating. These structures revealed that different from the attachment receptor CD55, binding of FcRn to the virions induces efficient release of “pocket factor” under acidic conditions and initiates the conformational changes in viral particle, providing a substantivestructural basis for understanding the mechanisms of enterovirus entry.
Dr. Xin Zhao, assistant professor from Institute of Microbiology, CAS; Dr. Guigen Zhang, postdoc from Beijing University; Sheng Liu, joint-supervision graduate student from University of Science and Technology of China and Institute of Microbiology, CAS; and Dr. Xiangpeng Chen, associate professor from Beijing Children’s Hospital, Capital Medical University, are co-first authors of this paper. Prof. George F. Gao, Prof. Wensheng Wei and Prof. Zhengde Xie are co-corresponding authors of this paper. This work was supported by funding from the Strategic Priority Research Program of CAS, the National Key Research and Development Program of China, the China National Grand S&T Special Project, the National Natural Science Foundation of China and others.
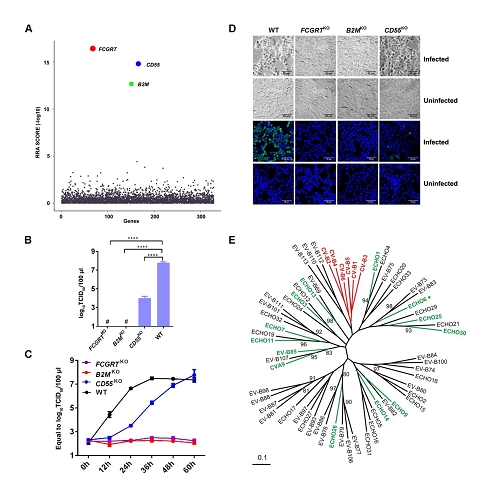
Figure 1: FcRn is a universal and essential receptor for EV-B entry

Figure 2: Cryo-EM structures of the Echo 6 viral particle and its complex with attachment (CD55) or uncoating (FcRn) receptor
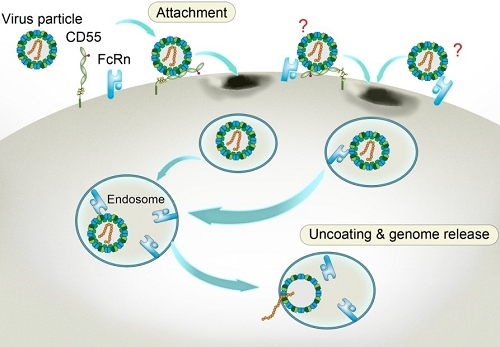
Figure 3: Model of echovirus entry into the cell
Contact:
Prof. GAO Fu
E-mail: gaof@im.ac.cn
CAS Key Laboratory of Pathogenic Microbiology and Immunology, Institute of Microbiology, Chinese Academy of Sciences, 100101, Beijing, China
