Nivolumab is a commercial monoclonal antibody (mAb) which has been approved by US FDA in 2014 to treat melanoma, non small cell lung cancer, etc. However, how this cancer drug works remains unclear.
Researchers from Prof. YAN Jinghua’s and Prof. George F. Gao’s groups at the Institute of Microbiology, Chinese Academy of Sciences resolved the complex structure of nivolumab with the ectodomain of PD-1 and showed that an unexpected N-terminal loop of PD-1 dominated the interaction with nivolumab. The interaction of nivolumab and PD-1 is independent of N-linked glycosylation. In addition, the binding epitopes of nivolumab to PD-1 are completely different from pembrolizumab, another clinically used antibody targeting PD-1.
Together, their study revealed molecular mechanisms of nivolumab-based PD-1-PD-L1 blockade. Glycosylation-independent interaction with nivolumab indicates a broader application of nivolumab in management of multiple tumors. The flexible N-loop, which dominated binding of PD-1 to nivolumab, deserves more attention for design of antibody-based drugs targeting PD-1 in the future.
Their work was published on Nature Communications (http://www.nature.com/cr/journal/vaop/ncurrent/full/cr2016102a.html).
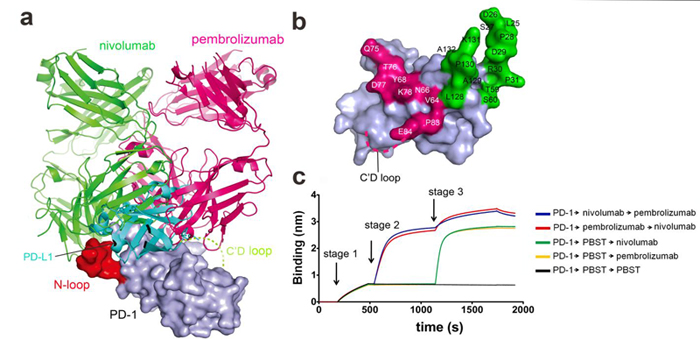
Distinct blockade binding mode of nivolumab compared with pembrolizumab (Image by Prof. YAN and Prof. GAO’s groups)
Contact:
Prof. YAN Jinghua
CAS Key Laboratory of Microbial Physiological and Metabolic engineering, IMCAS
E-mail: yanjh@im.ac.cn
Prof. George F. Gao
CAS Key Laboratory of Pathogenic Microbiology and Immunology,IMCAS
E-mail: gaof@im.ac.cn
摘要: Researchers from Prof. YAN Jinghua’s and Prof. George F. Gao’s groups at the Institute of Microbiology, Chinese Academy of Sciences resolved the complex structure of nivolumab with the ectodomain of PD-1 and showed that an unexpected N-terminal loop of PD-1 dominated the interaction with nivolumab.
关键词:nivolumab; structural mechanisms; blockade
