Antibody-based PD-1/PD-L1 checkpoint blockade therapies have taken center stage in immunotherapies for cancer, with multiple clinical successes. Checkpoint blockade takes advantage of a monoclonal antibody that blocks co-inhibitory signaling pathways to restore T-cell function, and subsequent elimination of tumors. Multiple PD-1/PD-L1 blockade antibodies have been approved by the US FDA for clinical use or have entered into clinical trials and have shown great efficacies to treat multiple advanced-stage tumors.
As one of the PD-L1 targeting antibodies, avelumab is a human IgG1 antibody with ADCC activity developed by Merck (Darmstadt, Germany) and Pfizer, which is now in multiple phase III clinical trials against non-small cell lung cancer, advanced renal cell cancer and gastric cancer, etc. Prof. George F. Gao's group has recently solved the crystal structural of avelumab complexed with human PD-L1 and clarified the structural basis of how PD-L1 targeting therapeutic antibodies blocks the PD-1/PD-L1 interaction.
Structural analysis of the interaction of avelumab with hPD-1 reveals that avelumab utilizes both VH and VL to bind to the IgV domain of PD-L1 on its side. The VH of avelumab dominates the binding to hPD-L1 by all three complementarity determining regions (CDR) loops, while VL contributes partial contacts by the CDR1 and CDR3 loops. The binding epitope region of avelumab on hPD-L1 predominantly consists of the C, C’, F, and G strands and the CC’ loop of hPD-L1. The detailed analysis of the buried surface on hPD-L1 reveals that the overlapping area of avelumab and hPD-1 is mainly located on the F and G strands, which are predominantly occupied by the HCDR2 loop of avelumab. Therefore, the mechanism of avelumab blockade involves the protruding HCDR2 loop dominating the hPD1 binding region and competing for the binding of hPD-1 to hPD-L1. The binding affinities (Kd) of avelumab to hPD-L1 is 42.1 pM while the binding affinity between hPD-1 and hPD-L1 is much weaker (0.77-8.2 μM). The strong binding of pembrolizumab to hPD-1 and avelumab to hPD-L1 would enable the binding priority of the therapeutic antibodies with checkpoint molecules and subsequent blockade of the hPD-1/hPD-L1 interaction.
This work was recently published on the journal of Cell Research. These findings would benefit the design and optimization of therapeutic antibodies targeting hPD-L1. Prof. George F. Gao's group has been focused on structural immunology for many years. Prof. George F. Gao's has achieved multiple progressions in researches into structural mechanisms of CD8- pMHC interaction (Nature, 1997, with front cover, etc.), TCR-pMHC interaction (Immunity,1999a;Immunity,1999b, etc.), MHC molecular evolution and rules of peptide presentation (multiple J Immunol, J Virol, etc.), OSCAR(PNAS, 2016)、PILR分子(PNAS, 2014) and ILT family molecules (J Biol Chem, 2011; J Mol Biol, 2009, etc.).
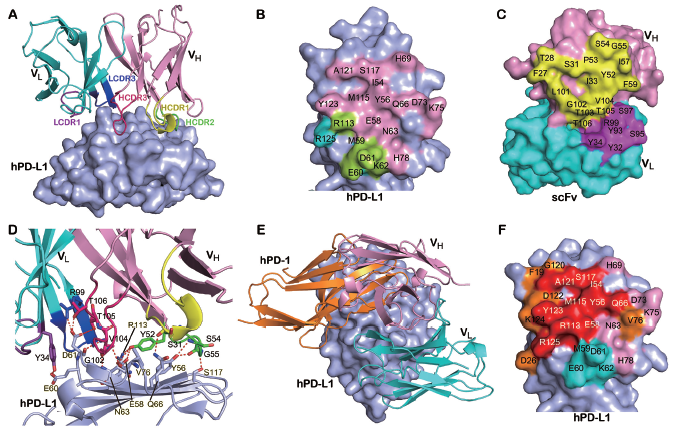
Detailed binding of avelumab to hPD-L1 for the blockade of hPD-1/hPD-L1 interaction.
link: http://www.nature.com/cr/journal/vaop/ncurrent/full/cr2016102a.html
