The recently reported Middle East respiratory syndrome coronavirus (MERS-CoV) is phylogenetically closely related to the bat coronaviruses (BatCoVs) HKU4 and HKU5.
The spike (S) protein in the MERS-CoV envelope is responsible for receptor binding and membrane fusion in viral entry. A previous report showed that the receptor binding domain (RBD) located in the S specifically engages its receptor, human CD26 (hCD26). High sequence identities (>50%) in both S and the RBD-homologous region observed between BatCoV HKU4/HKU5 and MERS-CoV raise the possibility that these two viruses may use hCD26 as a receptor.
Prof. GAO Fu (George F. Gao) and his colleagues at the Institute of Microbiology, Chinese Academy of Sciences (IMCAS) demonstrated that the RBD domain of BatCoV HKU4 (HKU4-RBD) but not the equivalent domain of BatCoV HKU5 (HKU5-RBD) can bind to hCD26. The binding affinity between HKU4-RBD and hCD26 was determined by surface plasmon resonance (SPR) within the micromolar range.
Despite the observed lower affinity compared to that between the MERS-CoV RBD (MERS-RBD) and hCD26, they found that pseudoviruses containing BatCoV HKU4 S can infect human Huh7 cells via hCD26.
They further solved the complex structure of HKU4-RBD bound to hCD26 to delineate the basis of receptor recognition. The complex structure shows an overall “U”-shaped structure. Similar to MERS-RBD, HKU4-RBD is composed of a core subdomain and an external receptor binding motif, which recognizes β-propeller of hCD26 (Figure a).
The structural analyses and the subsequent mutagenesis study further demonstrated a similar hCD26-binding mode between BatCoV HKU4 and MERS-CoV; but the details of variant amino acid interactions responsible for the differences in binding affinity are distinct.
Therefore, the results showed that HKU4 may infect host cells through recognition and binding to hCD26 (Figure b), indicating HKU4 has evolved to utilize hCD26 as a functional receptor and therefore gained one of the key factors sufficing for interspecies transmission and human infection. Moreover, the observed similar hCD26-binding mode between HKU4 and MERS-RBD supports the notion that MERS-CoV most likely originated from bats.
These results were published online in Cell Host& Microbe.
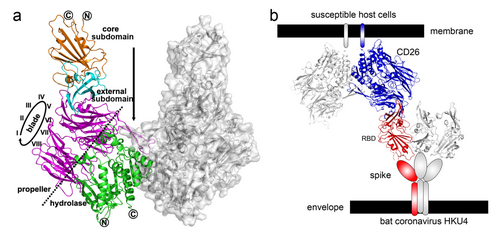
Figure. Complex structure of HKU4-RBD/hCD26 and a proposed binding model between BatCoV HKU4 and susceptible host cells (Image by Prof. GAO’s lab)
