Tuberculosis (TB) is a chronic and deadly infectious disease caused by M. tuberculosis (Mtb). About 10 million people have been estimated to have fallen ill with TB and 1.4 million people died from TB in 2019 (WHO, 2020). Mtb is an extremely successful intracellular pathogen that employs multiple effector proteins to manipulate host immune response, thereby promoting its infection, survival, pathogenicity and spread.
Dr. LIU Cuihua's group at the Institute of Microbiology, Chinese Academy of Sciences (IMCAS) has been investigating the molecular mechanisms underlying Mtb-host interactions, and her group has published a series of papers in top-ranked journals including Nature Immunology, Nature Communication, Proc Natl Acad Sci, EMBO reports, Cellular & Molecular Immunology. These studies have revealed the dynamic processes and the underlying molecular mechanisms of Mtb-host interactions, providing new strategies and potential specific targets for anti-TB therapeutics.
Mtb genome encodes for eleven eukaryotic-like serine-threonine protein kinases (STPKs), among which protein kinase G (PknG) is secreted into host cells and is essential for TB pathogenicity and pathogen intracellular survival during Mtb infection. Thus, PknG is a potentially attractive drug target for TB treatment.
A previous study from Dr. LIU's group previously demonstrated that PknG acts as an unusual ubiquitinating enzyme to promote the polyubiquitination and proteasomal degradation of host substrates (TRAF2 and TAK1) via a novel two-step cascade, leading to host innate immune suppression (EMBO reports, 2021).
More recently, Dr. Cui Hua Liu’s group further revealed another important novel mechanism by which Mtb PknG interferes with the host innate immunity. Basically, PknG targets different stages of the autophagy flux, including autophagy induction stage and autophagosome maturation stage, through distinct regions or its kinase activity, leading to an overall effect of blocked host autophagy flux and enhanced pathogen intracellular survival.
On the one hand, Mtb PknG suppresses the activation of AKT by exploiting its C-terminal region to competitively interact with the pleckstrin homology (PH) domain of AKT, thus promoting autophagy induction.
On the other hand, PknG also binds to RAB14 through its TPR domain, and phosphorylates AS160to suppress its GTPase-activating protein (GAP) activity towards RAB14 in a kinase activity-dependent manner, thereby keeping RAB14 in a RAB14-GTP state to prevent the maturation of Mtb-containing vesicles, thus promoting mycobacterial intracellular survival.
This study reveals a multi-functional bacterial effector that tightly regulates host autophagy flux to benefit pathogen intracellular survival during Mtb infection, and indicates a novel strategy adopted by Mtb to establish a sheltered niche (immature autophagosomes) to support the pathogen growth and to promote immune evasion.
It elucidates a novel mechanism by which Mtb induce autophagy through directly binding to AKT and thus inhibiting its kinase activity, strengthening our understanding towards the critical regulatory roles of PI3K-AKT-MTOR and phosphatidylinositol during pathogen-host interactions.
The study also identifies AS160 as a novel kinase substrate of PknG, and reveals the dual-regulatory roles of PknG towards autophagy through targeting RAB14: PknG binds to RAB14 through its TPR domain and blocks RAB14-bounded GTP hydrolysis and PknG can also phosphorylate AS160 to suppress its GAP activity towards RAB14.
In summary, this study reveals the whole picture of the PknG-mediated autophagy flux regulation during Mtb infection, providing potential TB treatment strategies through promoting PknG-AKT interaction to induce the autophagy while targeting the kinase activity of PknG and the PknG-RAB14 interacting interface to release the autophagy flux blockade effect (Figure 1).
The paper entitled “M. tuberculosis PknG manipulates host autophagy flux to promote pathogen intracellular survival” has been published online in Autophagy with GE Pupu and LEI Zehui as joint first authors and Dr. WANG Jing and Dr. LIU Cuihua as joint corresponding authors. This work was supported by grants from the National Natural Science Foundation of China, the Strategic Priority Research Program of the Chinese Academy of Sciences, the National Key Research and Development Program of China, the National Science and Technology Major Project and the Youth Innovation Promotion Association CAS.
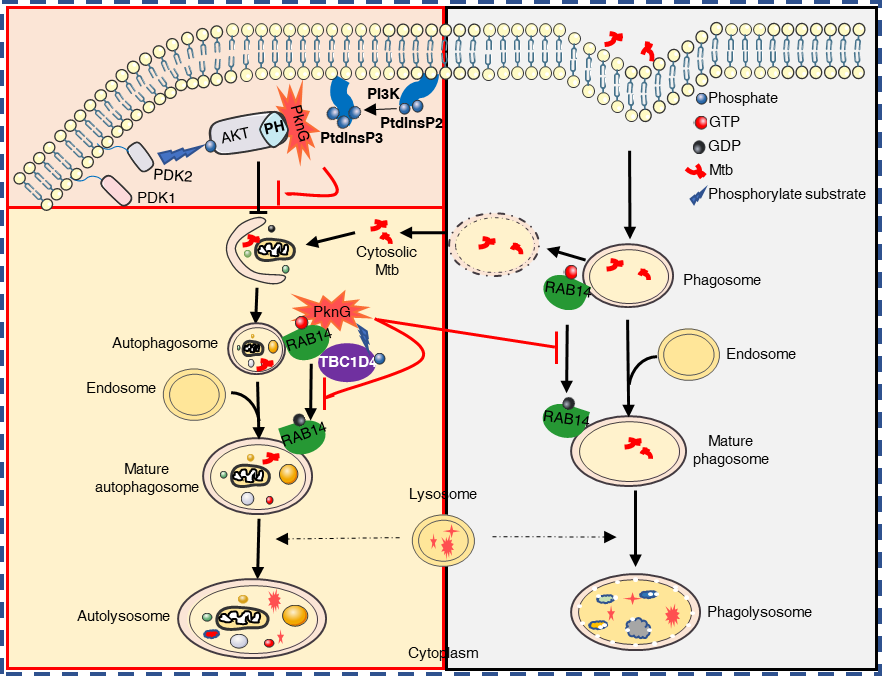
Figure 1. Proposed model depicting Mtb PknG-mediated host autophagy flux blockade through its multi-functional activity (Image by Dr. LIU Cuihua’s Group)
Access to this published article via this link: https://doi.org/10.1080/15548627.2021.1938912
