Tuberculosis (TB) is a chronic infectious disease caused by Mycobacterium tuberculosis (Mtb) and remains an intractable problem for human health. Dr. Cui Hua Liu's group has been investigating the molecular mechanisms underlying Mtb-host interplays, and previous studies from her group have provided new insights into the pathogenesis of TB as well as potential targets for the development of anti-TB treatments based on pathogen-host interacting interfaces (Nature Immunology, 2015; Nature Communications 2017, 2019; The Journal of Immunology 2015; Cellular Microbiology 2017; Cellular & Molecular Immunology 2018).
Besides causing TB, Mtb infection has long been associated with the development of both lung cancer and sarcoidosis. Internationally, lung cancer is the leading cause of cancer-related death in humans, among which lung adenocarcinoma (LUAD) is the most common histologic subtype. Sarcoidosis is a common autoimmune disease that preferentially affects the lungs and lymph glands, and has recently been linked to TB infection. However, the pathogenic links and molecular markers for the above three mentioned diseases have not been elucidated. More importantly, pulmonary TB could mimic lung cancer or sarcoidosis in some cases, which may challenge the diagnosis and delay the treatment. Recently, Dr. Cui Hua Liu's group revealed lung transcriptional signatures of pulmonary tuberculosis, adenocarcinoma and sarcoidosis. On the one side, Dr. Liu’s team found that TB shares a set of potential pathogenic mediators with LUAD or sarcoidosis, and further demonstrated that host MKI67, an over-expressed gene shared by TB and LUAD, is involved in Mtb-promoted tumor cell proliferation, migration and invasion. On the other side, they unraveled distinct lung modular signatures and molecular markers of TB, LUAD and sarcoidosis, andverified an ossification-related TB lung signature, which is probably associated with the activation of BMP/SMAD/RUNX2 pathway in Mtb-infected macrophages that controls mycobacterial survival and promotes osteogenic differentiation of mesenchymal stem cells. These findings provide new insights into Mtb pathogenesis and may contribute to differential diagnosis of TB, LUAD and sarcoidosis.
The paper entitled “Lung gene expression signatures suggest pathogenic links and molecular markers for pulmonary tuberculosis, adenocarcinoma and sarcoidosis” has been published online in Communications Biology with Qiyao Chai, Zhe Lu and Zhidong Liu being the co-first authors and Dr. Cui Hua Liu and Dr. Yu Pang being the co-corresponding authors. This work was supported by the National Key Research and Development Program of China, the National Natural Science Foundation of China, and the Strategic Priority Research Program of the Chinese Academy of Sciences.
Full text links: https://www.nature.com/articles/s42003-020-01318-0
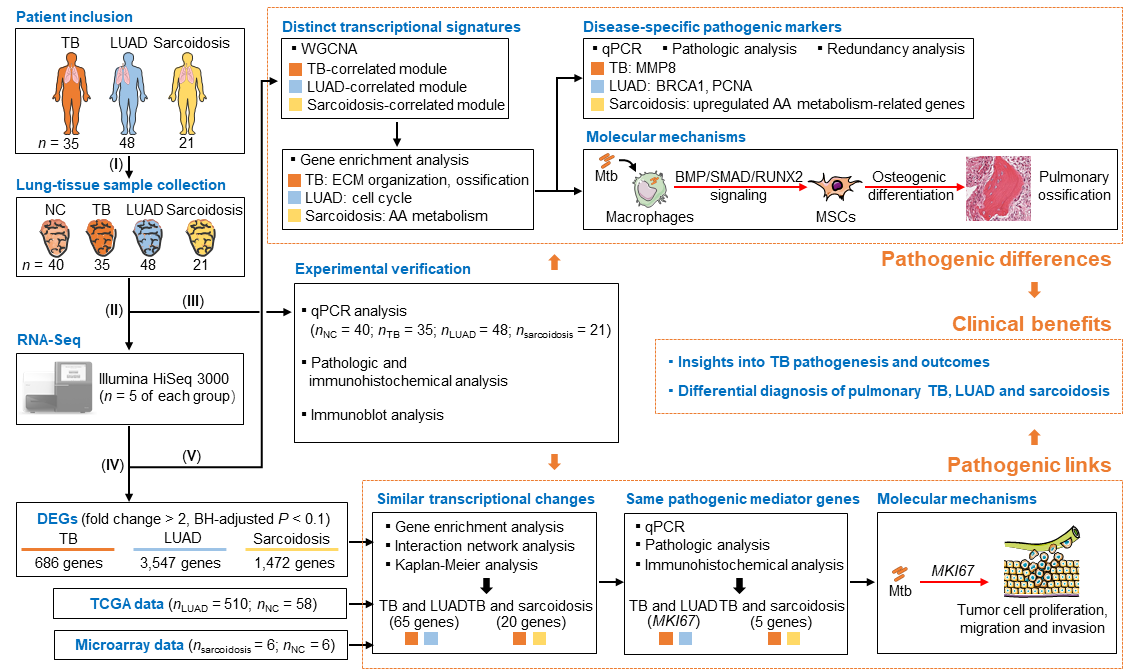
Figure 1. Lung transcriptome signatures reveal pathogenic links and molecular markers for pulmonary tuberculosis, adenocarcinoma and sarcoidosis
Contact:
Dr. Cui Hua Liu
E-mail: liucuihua@im.ac.cn
CAS Key Laboratory of Pathogenic Microbiology and Immunology, Institute of Microbiology, Chinese Academy of Sciences, 100101, Beijing, China
