Recently, Professor GAO Fu (George Fu Gao)’s group at Institute of Microbiology, Chinese Academy of Sciences, uncovered the virus entry mechanism of Enterovirus A by using the two-in-one receptor Kringle containing transmembrane protein 1 (KRM1). The article entitled “Molecular basis of Coxsackievirus A10 entry using the two-in-one attachment and uncoating receptor KRM1” was published in Proceedings of the National Academy of Sciences of the United States of America(PNAS)on July 20, 2020.
The family Picornaviridae represents a group of non-enveloped positive-strand RNA viruses. Within this family, the genus Enterovirus includes many important human pathogens that may cause severe diseases in humans and other mammals, e.g. the common cold, hand-foot-and-mouth disease (HFMD) and poliomyelitis.
As a causative agent for HFMD, Coxsackievirus A10 (CV-A10) poses great threats to the health of infants and young children worldwide. In addition to the symptoms of HFMD, CV-A10 infection can also lead to a broad range of clinical manifestations, such as fever, herpangina, and even developing viral meningitis.
Most enteroviruses enter host cells through a two-receptor mechanism, with a first (attachment) receptor to enable virion attaching at the cell surface and a second (uncoating) receptor to trigger conformational changes of viral particle which facilitates the genome release into the cytosol. Some enteroviruses, however, e.g. poliovirus, use only one receptor to accomplish the two steps of cell entry.
Previous studies have identified KRM1 as an essential receptor for CV-A10 entry, as well as several other enterovirus A species. However, the underlying mechanisms of KRM1-mediated viral entry have not been well understood.
George Fu Gao’s group presented a comprehensive mechanistic insight into CV-A10 infection using the receptor KRM1. They found that KRM1 could bind CV-A10 mature virion in both acidic and neutral pH conditions with high affinity, but not the empty viral particles.
Then they determined the atomic-resolution structures of CV-A10 alone and its complex with KRM1 at pH 7.4 and pH 5.5 by cryo-EM single particle reconstruction. These structures reveal that KRM1 selectively binds to the mature viral particle above the canyon of viral protein 1 (VP1) subunit and contacts across two adjacent asymmetry units. The key residues for receptor binding are conserved among most KRM1-dependent enteroviruses, suggesting a uniform mechanism for receptor binding.
Besides, the binding of KRM1 could induce efficient release of “pocket factor” under acidic conditions and accelerate the conformational change in viral particle to facilitate uncoating. They further reconstituted the uncoating process of CV-A10 by incubating the virion with KRM1 in vitro.
These evidences demonstrate KRM1 is a two-in-one attachment and uncoating receptor for CV-A10 infection, which mediates both viral attachment at the cell surface and uncoating in the endosome.
This work expands understanding on the mechanism of non-enveloped virus infection and indicates new clues for antiviral intervention.
CUI Yingzi from GUCAS is the first author of this paper. The study was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences.
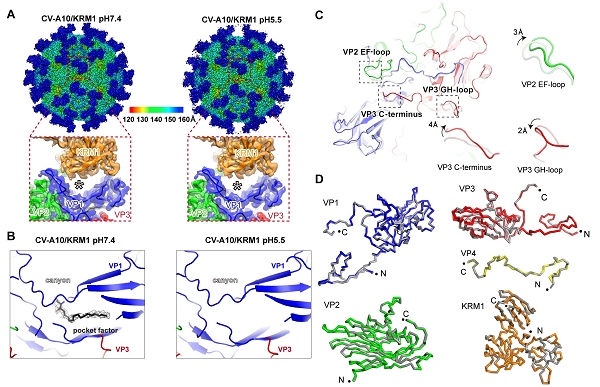
Fig. 1. KRM1-bound CV-A10 structures under neutral and acidic conditions (Image by Prof. GAO’s)
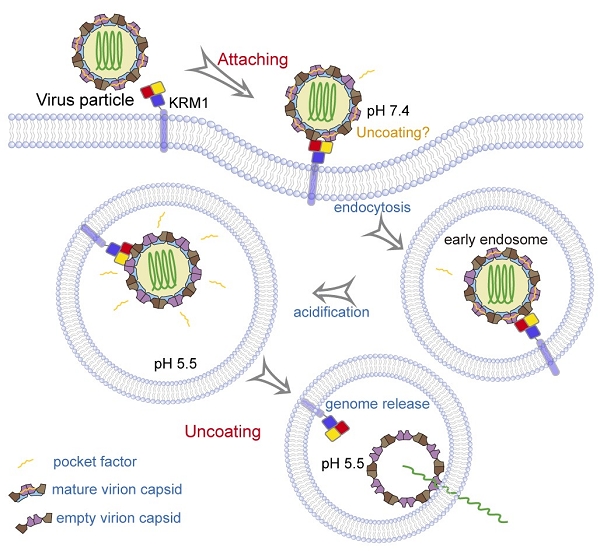
Fig. 2. KRM1 is a two-in-one receptor for CV-A10 entry into the cell (Image by Prof. GAO’s group)
