Tuberculosis (TB) is a chronic infectious disease caused by M. tuberculosis (Mtb) and remains a ticklish problem for human health. According to report from the World Health Organization (WHO), about 10.0 million people were estimated to have fallen ill with TB and 1.3 million people died from TB in 2017 (WHO, 2018). Mtb is a successful intracellular pathogen that employs multiple strategies to subvert host cellular functions for immune evasion, thus ultimately promoting their prolonged intracellular survival. On the other side, there is also a range of immune mechanisms adopted by the host to defend against invading mycobacteria. Such interaction and antagonism between the pathogen and the host continues throughout the whole period of Mtb infection, and has a profound impact on the course and outcome of TB disease.
Dr. Cui Hua Liu's group has been investigating the molecular mechanisms underlying Mtb-host interactions. Previous studies from her group revealed that a variety of effector proteins (such as PtpA and a number of Mce family proteins) delivered by Mtb could modulate host innate immunity to promote the intracellular survival of mycobacteria (Nature Immunology, 2015; Nature Communication, 2017; Cellular & Molecular Immunology, 2018; The Journal of Immunology, 2015; Cellular Microbiology, 2017). They also discovered a host restriction factor, TRIM27, which restricts intracellular survival of mycobacteria but is antagonized by Mtb PtpA (Scientific Reports, 2016). Those studies revealed the mutual antagonism between Mtb and host innate immune defense, and provided potential targets for the development of anti-TB treatments based on pathogen-host interacting interfaces.
Recently, it has emerged that ubiquitin-mediated xenophagy plays an important role in host immune defense against intracellular pathogens including Mtb. However, the exact mechanism by which host ubiquitin targets invaded microbes to trigger xenophagy remains vague. Previous studies from Liu's group found that the mycobacterial effector protein PtpA contains an ubiquitin-interacting motif-like (UIML) region for host ubiquitin binding and innate immune suppression, which discovery prompted them to wonder whether there are certain Mtb surface proteins that could directly bind to host ubiquitin for triggering xenophagy-mediated bacterial clearance. In their efforts to search for novel potential ubiquitin-binding proteins from Mtb, they identified a eukaryotic-like ubiquitin-associated (UBA) domain-containing Mtb surface protein Rv1468c. Interestingly, rather than being ubiquitinated by E3 ubiquitin ligases, Mtb Rv1468c could directly bind to host ubiquitin through UBA-dependent interaction, which is followed by recruitment of autophagy receptor p62, leading to the engulfment of mycobacteria into LC3-associated autophagosomes for Atg5-dependent autophagic clearance. Mutation of Rv1468c UBA domain to subvert its interaction with ubiquitin impairs host xenophagic clearance of Mtb in macrophages, and elevates bacterial loads in mice with enhanced inflammatory responses (Fig. 1). This study uncovers a previously unrecognized role of ubiquitin as an innate immune trigger that binds to the pathogen surface protein to evoke host antimicrobial autophagy, and indicates a novel “diplomatic” strategy adopted by Mtb to benefit its prolonged intracellular survival via maintaining optimized intracellular bacterial loads to avoid excessive host inflammatory responses.
The paper entitled “A Mycobacterium tuberculosis surface protein recruits ubiquitin to trigger host xenophagy” has been published online in Nature Communications with Qiyao Chai as the first author and Dr. Cui Hua Liu as the corresponding author. This work was supported by grants from National Basic Research Programs of China, the National Natural Science Foundation of China, and the Strategic Priority Research Program of the Chinese Academy of Sciences, etc.
Full text links: https://rdcu.be/bzs8B
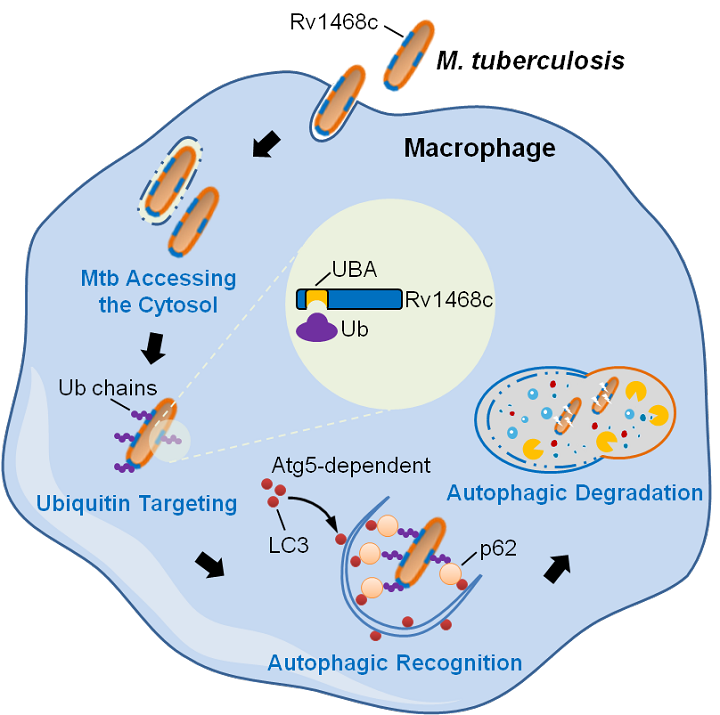
Figure 1. Schematic model showing mechanisms by which host ubiquitin directly binds to Mtb Rv1468c for triggering antibacterial xenophagy.
Contact:
Dr. Cui Hua Liu
E-mail: liucuihua@im.ac.cn
CAS Key Laboratory of Pathogenic Microbiology and Immunology, Institute of Microbiology, Chinese Academy of Sciences, 100101, Beijing, China
