Modulation of T-cell mediated anti-tumor immune responses with monoclonal antibodies (mAbs) has achieved brilliant clinical success. Because of the innovative findings related to CTLA-4 and PD-1, which lead to the discovery of mAb-based immune checkpoint therapy, James Allison and Tasuku Honjo have been awarded the 2018 Nobel Prize in Physiology or Medicine.
Cell Reports recently published a study about the molecular mechanisms of 4-1BB binding with its ligand 4-1BBL or the agonist monoclonal antibody utomilumab.
Co-stimulatory molecules play pivotal roles in T cell activation. 4-1BB (CD137, TNFRSF9), a member of TNFRSF, is a critical co-stimulatory checkpoint, which could inhibit T cell apoptosis, regulate T cell immune memory and differentiation. 4-1BB signal has been widely used in design of chimeric antigen receptor engineered T cells (CAR-T). Agonist mAbs targeting co-stimulatory 4-1BB, including urlumab (Bristol-Myers Squibb) and utomilumab (pfizer), have shown promising efficacy in treatment of multiple tumors when combined with other immunotherapies. (Figure).
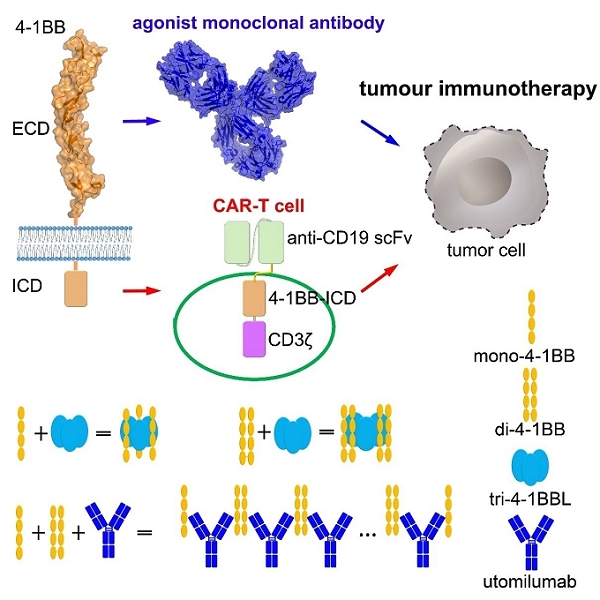
Figure. 4-1BB is a critical target for tumor immunotherapy (Image by Prof. George F. Gao’s team).
In this study, the complex structures of human 4-1BB with 4-1BBL ligand (4-1BBL) was determined which shows a typical TNF/TNFR binding mode. Three 4-1BBLs assemble as trimer (tri-4-1BBL) and bind three monomeric 4-1BBs (mono-4-1BB).
The complex structure of 4-1BB/utomilumab shows that the binding surface of 4-1BBL and utomilumab on 4-1BB overlapped with each other, indicating competitive binding of these two molecules. Competitive binding of 4-1BBL and utomilumab to 4-1BB was further confirmed with both cell-based flow cytometry assay and protein-based Octet assays.
The results showed that the binding of utomilumab to 4-1BB have blocked the binding of 4-1BBL, suggesting the interruption of 4-1BBL induced signaling.
Both monomeric (mono-4-1BB) and dimeric 4-1BBs (di-4-1BB) were detected to be expressed in activated T cells. Study on binding mode of 4-1BBL to di-4-1BB suggests that one tri-4-1BBL could bind to two di-4-1BBs and forms stable (di-4-1BB)2/4-1BBL complex, instead of forming aggerates, as predicted previously.
The limited cross-linking of di-4-1BB may be correlated with reduced threshold of T-cell activation, compared with mono-4-1BB alone. Alignment of the CRD4 domains of 4-1BBs from different species revealed that C121-mediated di-4-1BB formation is only present in primates and some of the other mammals, suggesting that the presence of di-4-1BB might be correlated with immune system evolution.
The binding modes of di-4-1BB and mono-4-1BB with 4-1BBL indicate restricted cross-linking of 4-1BBs. The findings of the molecular basis of 4-1BB interaction with both its ligand and utomilumab would be useful for future the development of biological agents targeting co-stimulatory 4-1BB for tumor immunotherapy.
The first authors of this paper are LI Yan and TAN Shuguang from the Institute of Microbiology, Chinese Academy of Sciences (IMCAS), and Chang Zhang from Suzhou Institute of Biomedical Engineering and Technology. The corresponding authors are Prof. George F. Gao and Po Tien from IMCAS, and Prof. Shan Gao from Suzhou Institute of Biomedical Engineering and Technology.
This work was supported by the National Natural Science Foundation of China and Strategic Priority Research Program of Chinese Academy of Sciences (CAS).
Reference:
Li Y#, Tan S#, Zhang C#, Chai Y, He M, Zhang C. W., Wang Q, Tong Z, Liu K, Lei Y, Liu W, Liu Y, Tian Z, Cao X, Yan J, Qi, J, Tien P*, Gao S*, Gao G.F.* Limited cross-linking of 4-1BB by 4-1BB ligand and the agonist monoclonal antibody utomilumab. Cell Rep. 2018. Oct 23;25(4):909-920.e4.
