Cyclophilin A (CypA, encoded by PPIA) is a peptidyl-prolyl cis/trans isomerase (PPIase) that is expressed ubiquitously in all type of cells. CypA is the major cellular target for the immunosuppressive drug cyclosporin A (CsA) and is involved in protein folding, cell signaling, inflammation, and tumorigenesis. Recent work suggests that it also plays a role in regulating virus replication.
Prof. LIU Wenjun’s group at the Institute of Microbiology, Chinese Academy of Sciences has been working on the function of CypA in regulating virus replication for many years. They have found that CypA-overexpressing transgenic mice exhibited resistance to influenza A virus infection.
Moreover, CypA interacted with influenza A virus M1 protein and inhibited virus replication by accelerating ubiquitin-proteasome degradation of the M1 protein, indicating that CypA can interact directly with viral protein to regulate virus replication. Yet several lines of evidence indicate that CypA can also regulate virus replication through modulating host immune responses. However, the molecular mechanism of how CypA regulates antiviral immune responses is rarely understood.
RIG-I can detect viral RNA and interact with MAVS to stimulate antiviral immune responses, leading to the production of type I interferons. Prof. LIU Wenjun’s group reveals an essential role of CypA in boosting RIG-I-mediated antiviral immune responses by controlling the ubiquitination of RIG-I and MAVS.
Deficiency of CypA impaired RIG-I-mediated type I IFN production and promoted viral replication in human cells and mice. Upon Sendai virus infection, CypA increased the interaction between RIG-I and its E3 ubiquitin ligase TRIM25, leading to enhanced TRIM25-mediated K63-linked ubiquitination of RIG-I that facilitated recruitment of RIG-I to MAVS. In addition, CypA and TRIM25 competitively interacted with MAVS, thereby inhibiting TRIM25-induced K48-linked ubiquitination of MAVS.
The article entitled “Cyclophilin A-regulated ubiquitination is critical for RIG-I-mediated antiviral immune responses” has been published online in eLife (https://elifesciences.org/articles/24425) with Associate Prof. SUN Lei and Prof. LIU Wenjun as corresponding authors, Dr. LIU Wei and Dr. LI Jing as joint first authors.
This work was supported by grants from the National Natural Science Foundation of China, the Key Research Program of the Chinese Academy of Sciences, and the National Key Technology Support Program. LIU is the principal investigator of the Innovative Research Group of National Natural Science Foundation of China.
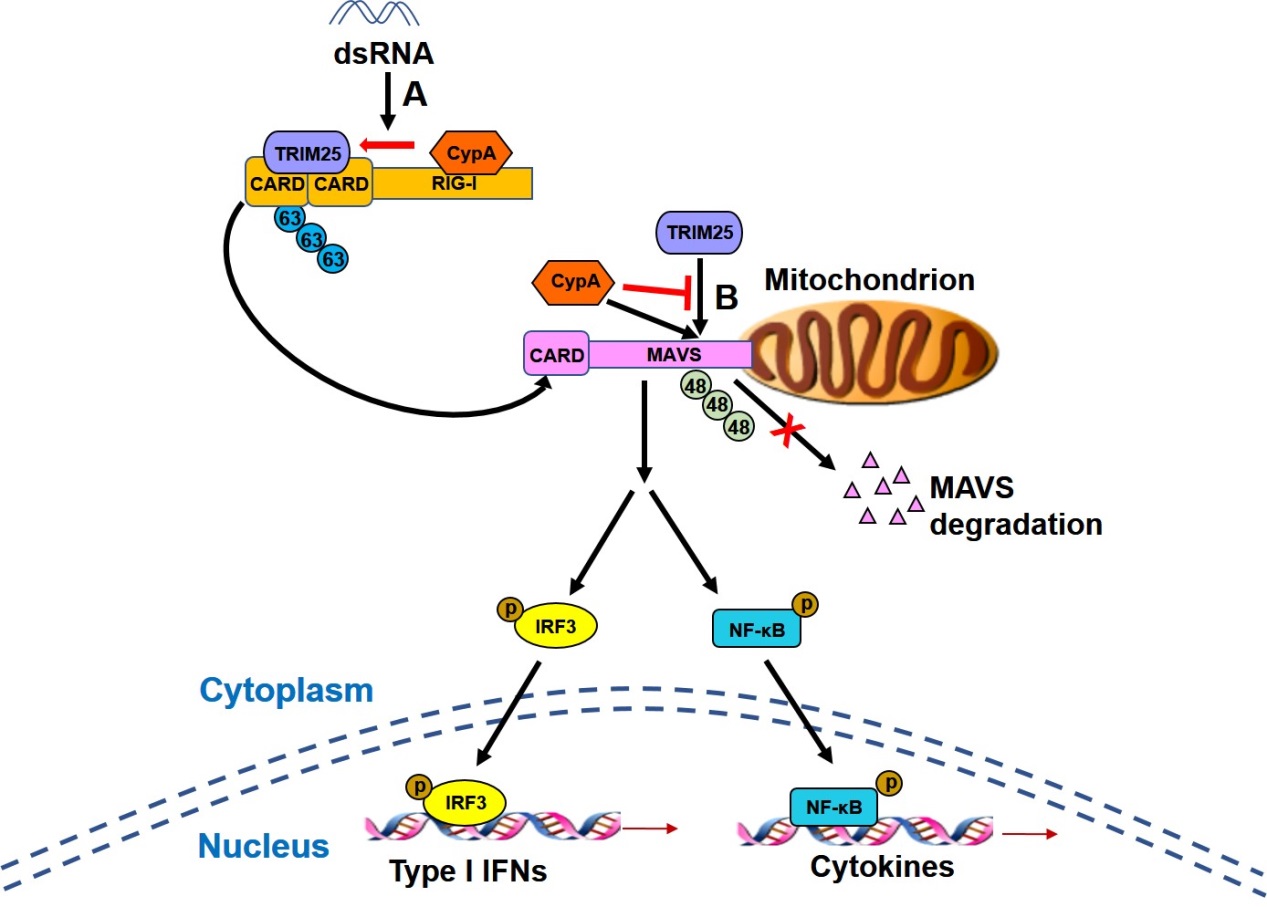
CypA boosts RIG-I-mediated antiviral immune responses. A. The binding of CypA to RIG-I-C promotes the interaction between TRIM25 and RIG-I-N, leading to enhanced TRIM25-mediated K63-linked ubiquitination of RIG-I that facilitates recruitment of RIG-I to MAVS. B. CypA and TRIM25 competitively interacts with MAVS, thereby inhibiting TRIM25-induced K48-linked ubiquitination of MAVS. (Image by Prof. LIU Wenjun’s group)
Contact:
SUN Lei
CAS Key Laboratory of Pathogenic Microbiology and Immunology,IMCAS
E-mail: sunlei362@im.ac.cn
LIU Wenjun
CAS Key Laboratory of Pathogenic Microbiology and Immunology,IMCAS
E-mail: liuwj@im.ac.cn
