In recent years, frequently emerging influenza A virus (IAV) epidemics have become a challenge to both veterinary and public health worldwide, such as the 2009 pandemic influenza A H1N1, avian influenza A H5N1, and H7N9. Influenza symptoms vary from mild disease to death; variations in influenza-induced disease severity in humans are usually attributed to the virulence of different influenza virus strains or the age or immune statues of infected individuals. However, the role of genetically determined host factors is less well understood.
The mouse has been shown to represent a particularly useful model to study the virulence of IAV infection, and previous studies have shown that the genetic background of mouse strains strongly influences the prognosis and severity after IAV infection. However, how the components of the host immune system, especially lymphocytes affecting on or contributing to the genetic related resistance or susceptibility after IAV infection are not known.
Dr. FANG’s group at the Institute of Microbiology, Chinese Academy of Sciences recently found that NK cells play differential roles against IAV infection, depending on the host genetic backgrounds. NK cells are large granular lymphocytes that mediate innate protection from some viral infections and tumor cells.
Several studies highlight the pivotal role of NK cells in the control of IAV infection in that defect in NK cell activity or depletion of NK cells result in delayed viral clearance and increased morbidity and mortality. However, there are also examples in which NK cells exacerbate morbidity and pathology during lethal dose influenza virus infection in mice, which indicate that the roles that NK cells play during influenza virus infection are intricate.
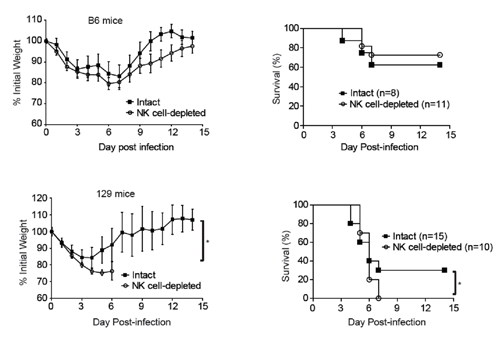
To investigate whether NK cells contribute to the genetic related resistance or susceptibility in different inbred mouse strains, the authors depleted NK cells in six inbred mouse strains, including C57BL/6, BALB/c, C3H, DBA/2, FVB and 129 mice.
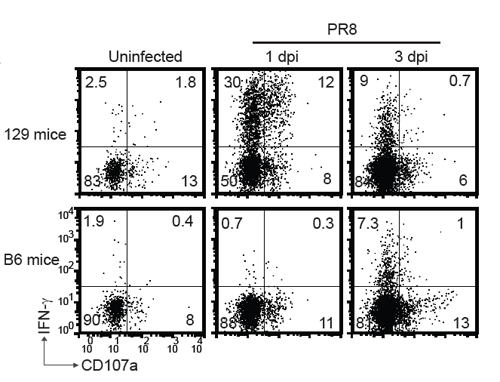
Surprisingly, NK cells played an important protective role only in 129 mice during high-dose IAV infection. Further mechanistic studies revealed that after high dose IAV infection, NK cells in 129 mice quickly activated and migrated to the infected lungs compared to that of C57BL/6 mice. The swift and strong NK cell responses efficiently controlled early pulmonary viral replication in 129 mice, providing survival privilege.
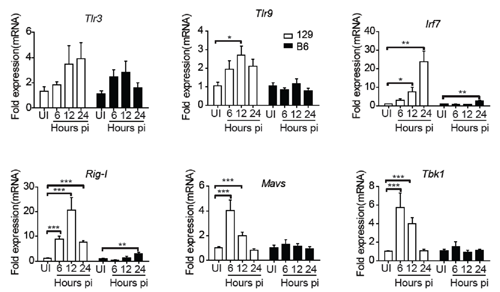
The authors also identified that the early activation of TLRs and RIG-I signaling at 1 day post infection(dpi) in 129 mice resulted in quick production of type 1 interferon and inflammatory cytokines, which might be important reasons for the swift kinetics of NK cell responses in 129 mice following high-dose IAV infection.
However, NK cells were not protective against low-dose IAV infection both in 129 mice and C57BL/6 mice. By measuring the NK cells responses and also the cytokine profiles in both mouse strains, the authors found that the kinetics of NK cell responses were similar between 129 mice and C57BL/6 mice. Further, no early cytokine production was found in both mouse strains at 1 dpi.
Thus, this work demonstrated that the responses and anti-viral functions of NK cells are affected by the host genetic backgrounds, and also the infecting viral doses. In 129 mice, the early induction of type 1 IFNs and inflammatory cytokines are important reasons for the strong and prompt NK cell responses.
This work links the kinetics and magnitudes of NK cell responses to the differential roles NK cells play during IAV infection. This finding also indicate that NK cell responses might be an important indicator for their function during viral infections, as NK cells are essential for some viral infections, while are dispensable during certain viral infections.
This demonstrates that the host genetic backgrounds fundamentally affect the kinetics and magnitudes of NK cell responses, thus related NK cell functions during IAV infection is unique. Further investigation for the molecular mechanisms that result in the differential activation of the pattern recognition receptors in 129 and C57BL/6 mice might identify new genetic related restriction factors for severe influenza.
This work was supported by National Basic Research Program (973) of China (2014CB542602, 2015CB910503), National Natural Science Foundation of China (Grant No. 31322020, 31370877), and intramural grant of the Chinese Academy of Sciences (KJZD-EW-L09-3).
High-dose IAV infection resulted in swift and strong NK cell responses in 129 mice
(Image from Dr. FANG’s lab).
Key Words: influenza virus; NK cells; host genetics; type 1 interferon
Article website:http://www.jimmunol.org/cgi/pmidlookup?view=long&pmid=26773146
Contact:
Dr. FANG Min
CAS Key Laboratory of Pathogenic Microbiology and Immunology,Institute Of Microbiology,Chinese Academy of Sciences,100101,Beijing, China
E-mail: fangm@im.ac.cn
