The research group led by Dr. Cui Hua Liu from the Institute of Microbiology, Chinese Academy of Sciences has been focused on studying the molecular mechanisms of pathogenic bacteria-host interactions, using Mycobacterium tuberculosis as the main research model. In a previous study published as a cover story in Nature Immunology in early March this year, Dr. Liu’s group revealed that M. tuberculosis suppresses innate immunity by coopting the host ubiquitin system (Nature Immunology, 2015, 16: 237-245.). In this new work, they further discover a novel mechanism by which M. tuberculosis Mce3E suppresses host innate immune responses. The related paper titled “Mycobacterium tuberculosis Mce3E suppresses host innate immune responses by targeting ERK1/2 signaling” was recently published online by The Journal of Immunology.
The majority of the currently available anti-tuberculosis drugs are ineffective or only partially effective due to the impermeable nature of the mycobacterial cell wall and the propensity of M. tuberculosis to develop drug resistance. Therefore, there is an urgent need to identify new therapeutic targets for developing drugs that can treat drug-susceptible and drug-resistant tuberculosis. M. tuberculosis can escape from immune surveillance so as to survive inside the host for long periods of time, causing latent infection, but the underlying molecular mechanisms remain largely unknown. There are increasing numbers of examples of bacterial pathogens that produce and secrete effector molecules that dampen inflammatory cytokine production. But the molecular mechanisms by which specific mycobacterial effectors modulate the host innate immune signaling pathways remain largely undefined. Results from such studies could provide a better understanding of the pathogenesis of tuberculosis, as well as to identify novel anti-tuberculosis drug targets.
The genome of M. tuberculosis contains four copies of a cluster of genes termed mammalian cell entry (mce) operons, all of which code for putatively exported proteins. The Mce proteins can specifically engage in small molecular compounds and are absent from human genome. Therefore, they might represent ideal candidate drug targets. The mammalian cell entry protein 3E (Mce3E), encoded by the third operon of mce (mce3), is located in the region of difference 15 of the M. tuberculosis genome and absent in mycobacterium bovis bacillus Calmette-Gue´rin (BCG), has an essential role in facilitating the internalization of mammalian cells by mycobacteria. However, relatively little is known about the role of Mce3E in modulation of host innate immune responses. In this study, the researchers demonstrate that Mce3E inhibits the activation of the ERK1/2 signaling pathway, leading to the suppression of Tnf and Il6 expression, and the promotion of mycobacterial survival within macrophages. Mce3E interacts and colocalizes with ERK1/2 at the endoplasmic reticulum in a DEF motif (an ERK-docking motif)–dependent manner, relocates ERK1/2 from cytoplasm to the endoplasmic reticulum, and finally reduces the association of ERK1/2 with MEK1 and blocks the nuclear translocation of phospho-ERK1/2. Inhibition of the ERK1/2 pathway in macrophages using U0126, a specific inhibitor of the ERK pathway, also leads to the suppressed Tnf and Il6 expression and the enhanced intracellular survival of mycobacteria. Taken together, these results suggest that M. tuberculosis Mce3E exploits the ERK1/2 signaling pathway to suppress host innate immune responses, providing a potential Mce3E–ERK1/2 interface–based drug target against M. tuberculosis.
Graduate student Jie Li from Prof. Cui Hua Liu’s lab is the first author. Prof. Cui Hua Liu is the corresponding author. The research was supported by the National Basic Research Programs of China, the National Natural Science Foundation of China, the Ministry of Health and the Ministry of Science and Technology of China, and the Chinese Academy of Sciences, and carried out at the Institute of Microbiology, Chinese Academy of Sciences, Beijing.
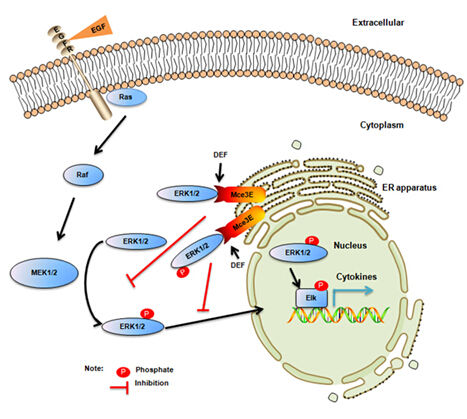
Figure: Proposed model for the mechanism of M. tuberculosis Mce3E-mediated host innate immune suppression. Mce3E interacts with and colocalizes with ERK1/2 at the ER apparatus in a DEF motifdependent manner. Through association with ERK1/2, Mce3E changes the subcellular localization of ERK1/2 from cytoplasm to the ER apparatus, reduces the association of ERK1/2 with MEK1, and blocks the nuclear translocation of phospho-ERK1/2, leading to inhibition of ERK1/2 signaling pathway activation, suppression of Tnf and Il6 expression, and promotion of mycobacterial survival within macrophages. (Image by Jie Li and Cui Hua Liu)
http://www.jimmunol.org/content/early/2015/03/14/jimmunol.1402679.long
CONTACT:
Cui Hua Liu
CAS Key Laboratory of Pathogenic Microbiology and Immunology, Institute of Microbiology, Chinese Academy of Sciences, Beijing 100101, China.
Email address: liucuihua@im.ac.cn
