Streptothricins are atypical aminoglycoside antibiotics possessing antibacterial and antifungal activities. ZhongShengJunSu, a product of streptothricins, is widely used as agriculture antibiotic for crop protection and so on. A defect in this product is it contains various streptothricin components, and which makes the product quality control very difficult.
The CHEN Yihua’s lab in State Key Laboratory of Microbial Resources, Institute of Microbiology, Chinese Academy of Sciences is devoting to understanding the biosynthetic mechanism of streptothricins, manipulating their biosynthetic pathway and improving the related product qualities. In this study, they elucidated the biosynthetic pathway of the D-gulosamine moiety and found that two novel enzymatic catalysts were involved in this process. These data were published on “Angew. Chem. Int. Ed.”online recently.
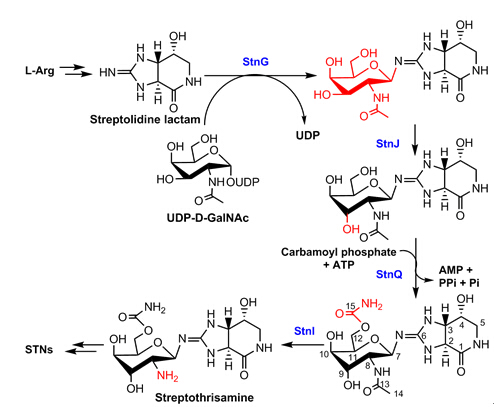
The biosynthesis of the rare sugar moiety D-gulosamine is depicted as the following figure. At first, a glycosyltransferase StnG adds a D-GalNAc from UDP-D-GalNAc to the guanidino-imine of streptolidine; StnJ catalyzes the epimerization of the 9-OH; StnQ is a carbamoyltransferase installing a carbamoyl group at 12-OH; finally, StnI remove the acetyl group from 8-NH to form 12-carbamoyl-streptothrisamine. StnG is an unprecedented GT-A fold guanidino-imine glycosyltransferase and StnI represents a novel type of LmbE acetyltransferase recognizing non-D-GlcNAc substrates.
Dr. GUO Zhengyan, Dr. LI Jine and Dr. QIN Hua contributed equally to this work. The research was supported by the MOST of China, NSFC and CAS.
http://onlinelibrary.wiley.com/doi/10.1002/anie.201412190/abstract
