Upon stimulated by environmental signals, the expression levels of responsive genes of organisms have to be changed accordingly for long-term survival. This process is far more than a simple “on-off” switch, but has a subtle pattern that is subjected to tight regulation.
Among the known kinetic patterns, expression surge was generally observed, such as in eliciting bacterial virulence factor expression to defend against the host immune attack, producing hormone during animal development, and expressing tumor necrosis factor (TNF) along with tumorigenesis. Yet currently little is known about the molecular mechanisms under expression surge regulation.
Known as the “nervous system” of prokaryotes, two-component signal transduction system (TCSTS) is the most important mechanism employed by bacteria to sense and respond to environmental stimuli. A typical TCSTS consists of a histidine kinase (HK) and a response regulator (RR), which carries out transmembrane signal transduction by phospho-transfer from the autophosphorylated HK to its cognate RR.
Bacterial genomes usually encode several to hundreds of TCSTS proteins, whereas this number is considered to be the bacterial “IQ” that reflects adaptive potential of the organism. In a plant bacterial pathogen, Xanthomonas campestris pv. campestris (Xcc), up to 106 TCSTS protein-coding genes were identified in its genome, indicating that Xcc is a high “IQ” organism among gram-negative bacteria.
Based on comparative and functional genomic analyses of the TCSTSs encoded by Xcc, researchers from the Institute of Microbiology, Chinese Academy of Sciences (IMCAS), identified a hybrid HK SreS. This HK has a very special secondary structure since that it contains two additional RR-like domains besides the conservative HK domain.
Interestingly, under high salt stress, rather than phosphorylating the RR SreR, SreS behaves as a phosphate sink to compete with SreR by its N-terminal receiver domain to rob the phosphoryl group from SreK, the cognate HK of SreR. This competition resulted in dephosphorylation of SreR.
Subsequently, the unphosphorylated SreR activates one of the two tandem promoters of the sreK-sreR-sreS-hppK operon to elicit the expression surge of HPPK, an essential enzyme required in folic acid de novo biosynthesis pathway.
The expression surge of HPPK was proved to be absolutely necessary for bacteria to resist high salt stress. Researchers then concluded that this dephosphorylation-dependent expression surge is a novel identified regulatory mechanisms for bacteria to respond to environmental clues. The “three-component” signal transduction system containing SreK, SreR and SreS which is more complex than traditional TCSTS. The complicated nature confers it more flexible roles during fine-tune of cellular processes.
Dissecting the structure, regulatory function and molecular evolution of the TCSTS are one of the research interests of Prof. QIAN Wei′ s laboratory in IMCAS. The above project was mainly achieved by Ms. WANG Fangfang, a Doctoral student of IMCAS, and was supported by grants from the National Natural Science Foundation of China. This work has been published online in Environmental Microbiology.
Author for correspondence: Prof. QIAN Wei (qianw@im.ac.cn; Tel: +86-10-64806063)
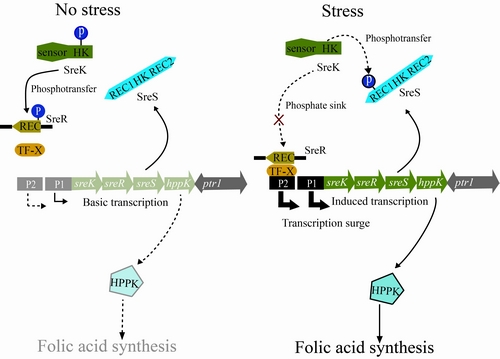
Working model of the three-component signaling system SreK-SreR-SreS during bacterial response to stresses (Image by Prof. QIAN's lab)
