Chronic hepatitis B (CHB)is a disease that has a huge impact on global public health as it is correlated with a significant increased risk for the development of cirrhosis, liver failure and hepatocellular carcinoma (HCC).However, until now the mechanisms for viral evasion of host defense and pathogenesis of HBV infection are still unclear. Information regarding the balance between viral parameters and host defense factors is essential to understand the molecular basis for viral pathogenesis, which may facilitate the development of novel targeted agents with improved therapeutic efficiency.
The “Hepatitis B immunology” group led by Prof. MENG Songdong in Institute of Microbiology,Chinese Academy of Sciences (IMCAS) investigated the potential role and mechanism of miR-122 in regulating HBV replication, which is the most abundant liver-specific microRNA in liver. They found that miR-122 expression in liver was significantly down-regulated in patients with HBV infection compared with healthy controls, and the miR-122 levels were negatively correlated with intrahepatic viral load and hepatic necroinflammation. miR-122 significantly inhibits HBV expression and replication. Further study showed that loss of miR-122 expression leads to up-regulation of its target cyclin G1, thus interrupts interaction between cyclin G1 and p53, and abrogates p53-mediated inhibition of HBV replication.
Their work demonstrates that miR-122 down-regulation induced by HBV infection can impact HBV replication and possibly contribute to viral persistence and carcinogenesis. Moreover, as miR-122 has been shown to play an important role in hepatic function and act as a tumor suppressor, this study suggests that induction and increase of miR-122 expression may provide an effective strategy for treatment of HBV infection and related liver diseases.
This work,funded by National Program on Key Basic Research Project of China (973 Program)and the National Natural Science Foundation of China, will be published in “Hepatology” (doi: 10.1002/hep.24809)
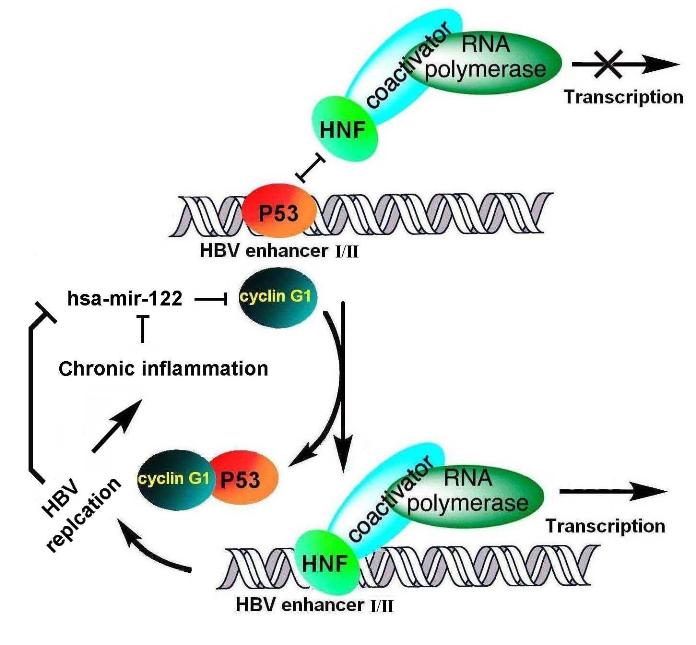
The mechanism of miR-122 mediated HBV suppression by modulation of p53 through down-regulation of cyclin G1. According to this model, miR-122 acts as a host restriction factor in HBV infection, and its down-regulation during HBV infection might contribute to viral persistence. (Image by IMCAS)
