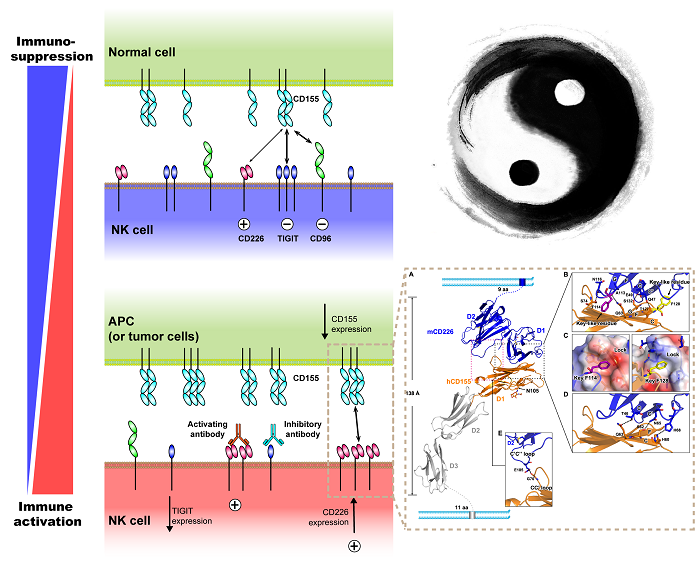
Figure1. Interaction of CD226 and CD155 plays a pivotal role in NK cell activation (Image by Prof. Gao’s group)
Moreover, they systematically analyzed the binding mechanism between CD226 and CD155 by determining a hybrid complex structure of mouse CD226 bound to CD155, which revealed a conserved binding interface within the first domain of CD226 (D1). Intriguingly, they found that the second domain of CD226 (D2) both provides structural supports for the unique architecture of CD226 and forms direct interactions with CD155. This distinct binding model is unique in TIGIT-CD226-CD96 paired receptor family.
Taken together, the structural and functional data provided by Prof. George Fu Gao’s group have broadened our knowledge of the recognition between NK cell receptors and the nectin/Necl family ligands and provide molecular basis for the development of CD226-targeted antitumor immunotherapy.
This research was funded by National Natural Science Foundation of China (NSFC, Grant No. 31390432, and 31700149), Strategic Priority Research Program of the Chinese Academy of Sciences (Grant XDB29010000), and National Postdoctoral Program for Innovative Talents (Grant BX201600162).