Natural killer (NK) cells are important component of innate immunity for the roles in responding early to viral infection and malignancies by cell-mediated cytotoxicity, and also contribute to activating and reshaping the adaptive immune responses via secreted cytokines. The functions of NK cells are regulated by various cell-surface expressed receptors, which can be grouped into stimulatory or inhibitory receptors according to the signals they transduced.
Recently, a paired NK cell receptor family, which specifically recognizes the nectin and nectin-like (Necl) family members, was discovered. These emerging receptors are activating receptors DNAM-1(also known as CD226), as well as inhibitory receptors TIGIT and CD96. These receptors can modulate NK cells functions by recognizing a common ligand, Necl-5 (also known as CD155). As the only activating receptor in this paired receptor family, CD226 are proved to play pivotal roles in modulating NK cell adhesion and cytotoxicity, facilitating immunological synapse formation and promoting cytokine secretion during inflammation.
In an article entitled “Binding mode of the side-by-side two-IgV molecule CD226/DNAM-1 to its ligand CD155/Necl-5”, researchers, led by Prof. George Fu Gao, from Chinese Academy of Sciences reported the structures of human and mouse CD226, both of which present a unique side-by-side arrangement pattern of two tandem immunoglobulin V-set (IgV) domains. This arrangement mode is distinct from the conventional head-to-tail organizing mode, as shown in the structure of CD155, of all the known Ig-like superfamily members. The only exception they found is VCBP3, an Ig-like molecule in amphioxus that diverged early in phylogeny. This result might give a hint of the evolution of Ig-like molecules.
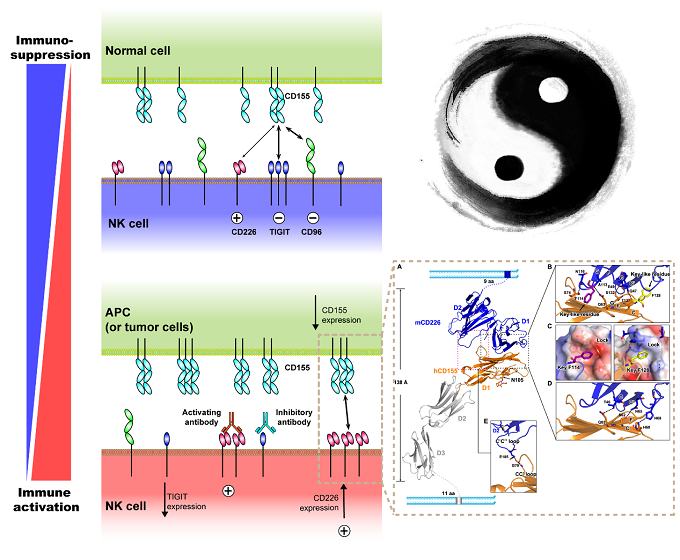
Figure1. Interaction of CD226 and CD155 plays a pivotal role in NK cell activation
Moreover, they systematically analyzed the binding mechanism between CD226 and CD155 by determining a hybrid complex structure of mouse CD226 bound to CD155, which revealed a conserved binding interface within the first domain of CD226 (D1). Intriguingly, they found that the second domain of CD226 (D2) both provides structural supports for the unique architecture of CD226 and forms direct interactions with CD155. This distinct binding model is unique in TIGIT-CD226-CD96 paired receptor family.
Taken together, the structural and functional data provided by Prof. George Fu Gao’s group have broadened our knowledge of the recognition between NK cell receptors and the nectin/Necl family ligands and provide molecular basis for the development of CD226-targeted antitumor immunotherapy.
This research was funded by National Natural Science Foundation of China (NSFC, Grant No. 31390432, and 31700149), Strategic Priority Research Program of the Chinese Academy of Sciences (Grant XDB29010000), and National Postdoctoral Program for Innovative Talents (Grant BX201600162).
See the article: Wang, H., Qi, J., Zhang, S., Li, Y., Tan, S., and Gao, G.F. (2018). Binding mode of the side-by-side two-IgV molecule CD226/DNAM-1 to its ligand CD155/Necl-5. Proceedings of the National Academy of Sciences of the United States of America. https://doi.org/10.1073/pnas.1815716116