Dr. Cui Hua Liu's group (from the Institute of Microbiology, Chinese Academy of Sciences) has been investigating the molecular mechanisms underlying pathogen infection and immune regulation. In recent years, Liu's group has published a series of papers in top-ranked journals including Nature Immunology, Molecular Cell, Nature Communication, Autophagy, Proc Natl Acad Sci, Cellular & Molecular Immunology and EMBO reports.
Inflammatory bowel diseases (IBDs) including Crohn’s disease (CD) and ulcerative colitis (UC) are complex multifactorial diseases, characterized by chronic and relapsing inflammation and disorganized immune responses in the gastrointestinal tract. In the recent decades, the incidence of IBD is rising worldwide including highly populated countries like China and there is a lack of effective prevention and treatment measures clinically. Intestinal homeostasis is maintained by complex regulatory mechanisms involving in a variety of immune cells, and dysregulation of intestinal immune system may cause inflammatory diseases and tumorigenesis. Thus, investigating the precise regulatory mechanisms that control immune responses in the intestinal microenvironment will provide new strategies for therapeutic intervention of IBDs. Ovarian tumor deubiquitinase 1 (OTUD1), as a key deubiquitinase, has been reported to be involved in the regulation of autoimmune diseases, viral and fungal infections and intestinal carcinogenesis. However, the function and regulatory mechanism of OTUD1 in intestinal inflammation remain unknown.
Recently, Dr. Cui Hua Liu’s group, in collaborating with Dr. Lingqiang Zhang (from National Center for Protein Sciences, Beijing), have revealed that OTUD1 interacts with receptor-interacting serine/threonine protein kinase 1 (RIPK1) and deubiquitinates K63-linked polyubiquitin chains of RIPK1 at lysine 627, which inhibits the recruitment of NF-κB essential modulator (NEMO) and the activation of NF-κB signaling pathway, leading to the suppression of proinflammatory cytokines production and intestinal inflammation (Figure 1). Firstly, this study shows that the expression of OTUD1 is upregulated upon dextran sulfate sodium (DSS) administration and loss of Otud1 in mice contributes to the pathogenesis of DSS-induced colitis with excessive release of proinflammatory cytokines, including TNF-α, IL-6 and IL-1β. Then, bone marrow transplantation experiments reveal that OTUD1 in hematopoietic cells plays a dominant role in the protection against colitis. Mechanistically, LPS-induced hypomethylation of OTUD1 promoter region facilitates its upregulation. OTUD1 subsequently interacts with receptor-interacting serine/threonine-protein kinase 1 (RIPK1) and selectively cleaves the K63-linked polyubiquitin chains from RIPK1 to inhibit the recruitment of NF-κB essential modulator (NEMO). Moreover, the expression of OTUD1 in the mucosa samples of ulcer colitis (UC) patients is lower than that in healthy controls. Finally, this study demonstrates that UC-associated OTUD1 G430V mutant abolishes its ability to inhibit RIPK1-mediated NF-κB activation and intestinal inflammation.
In summary, the findings from this study reveals that deubiquitinase OTUD1 exhibits an inhibitory effect on RIPK-mediated NF-κB activation to prevent intestinal inflammation through specially removing the K63-linked ubiquitin chains of RIPK1, suggesting that the immune intervention targeting OTUD1-RIPK1 axis could be an efficient therapy for IBD, which might provide novel potential therapeutic target for the treatment of IBD.
Full text links: https://www.nature.com/articles/s41423-021-00810-9
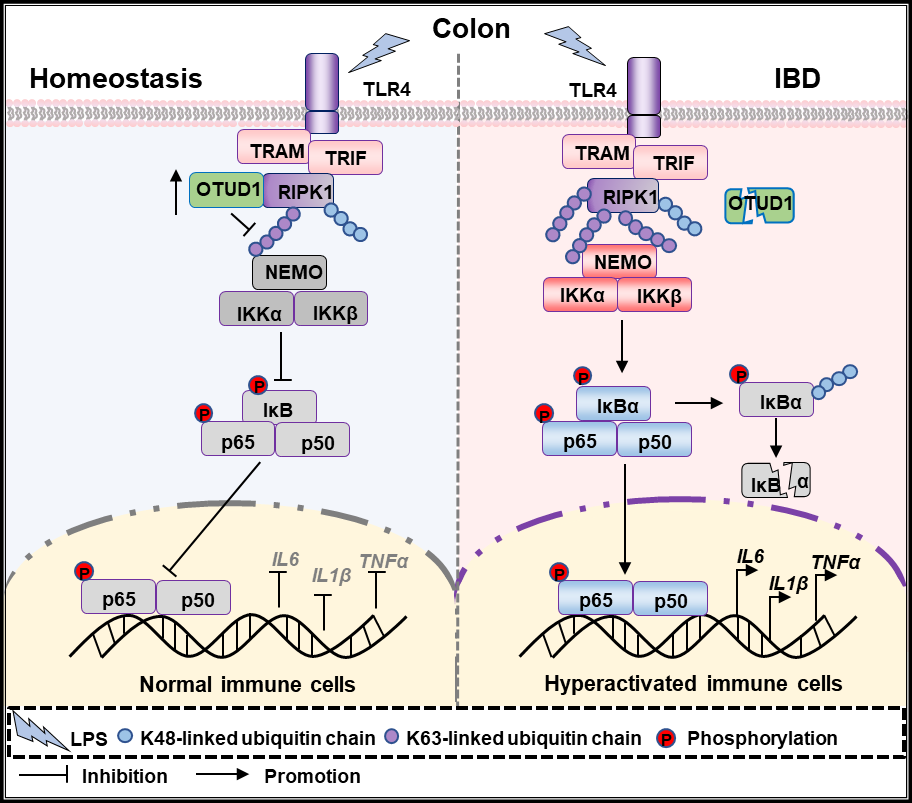
Figure 1. A proposed model that the deubiquitinase OTUD1 inhibits DSS-induced colitis through suppressing RIPK1-mediated NF-κB pathway. The paper entitled “The deubiquitinase OTUD1 inhibits colonic inflammation through suppressing RIPK1-mediated NF-κB signaling” has been published online in Cellular & Molecular Immunology with Dr. Bo Wu, Lihua Qiang, Yong Zhang and Yesheng Fu as joint first authors, Dr. Cui Hua Liu and Dr. Lingqiang Zhang as joint corresponding authors. Dr. Hui Zheng (from Institutes of Biology and Medical Sciences, Soochow University) provides Otud1-/- mice for this work. This work is jointly supported by the Biosafety Special Project of China, the National Natural Science Foundation of China, the Strategic Priority Research Program of the Chinese Academy of Sciences and the State Key Laboratory of Proteomics. Contact: Dr. Cui Hua Liu E-mail: liucuihua@im.ac.cn CAS Key Laboratory of Pathogenic Microbiology and Immunology, Institute of Microbiology, Chinese Academy of Sciences, 100101, Beijing, China