A research team led by Prof. GAO George and TAN Shuguang from the Institute of Microbiology of the Chinese Academy of Sciences recently reported the unique binding mode of the T cell costimulatory receptor glucocorticoid-induced tumor necrosis factor receptor family-related protein (GITR) with its ligand GITRL for T cell activation. The results were published online in Cell Reports.
GITR is a critical molecule involved in regulation of T cell reactivity and agonistic monoclonal antibodies targeting GITR have shown promising efficacy in tumor immunotherapy in clinical investigations. GITR and its ligand GITRL is a paired member of TNF/TNFR superfamily. It has been broadly accepted that the receptor-ligand binding mode is highly conserved among TNF/TNFR superfamily members, e.g. a TNFR molecule binds to the side cleft formed by two adjacent TNFs. However, the GITR/GITRL complex structure and mutational analysis reported in this study reveal that the binding interface between GITR/GITRL is atypical in TNF/TNFR superfamily members.
The fact that one single protomer in GITRL mediates its binding with the single domain in the receptor indicates that such interaction may be the ancestral mode in the evolution of the TNF/TNFR superfamily, whereas the other TNFR superfamily members have evolved to engage with specialized multi-domains to form high-affinity complexes. The “D93-I94-V95” (DIV) in mouse GITR and the corresponding “K105-F106-S107” (KFS) in human GITR determine the binding specificities of the receptors to their ligands. The “DIV-to-KFS” swapped mutation of mouse GITR also resulted in activating signal transduction upon cross-recognition with human GITRL. The unique assembly of GITR/GITRL indicates a distinct intracellular receptor cytoplasmic signaling domain recruitment mechanism.
These findings reported in this study would not only expand the understanding of the interactions of TNF/TNFR superfamily molecules, but also shed light for the future design of drugs targeting GITR/GITRL.
This study was supported by projects from the Chinese Academy of Sciences and the Ministry of Science and Technology of China.
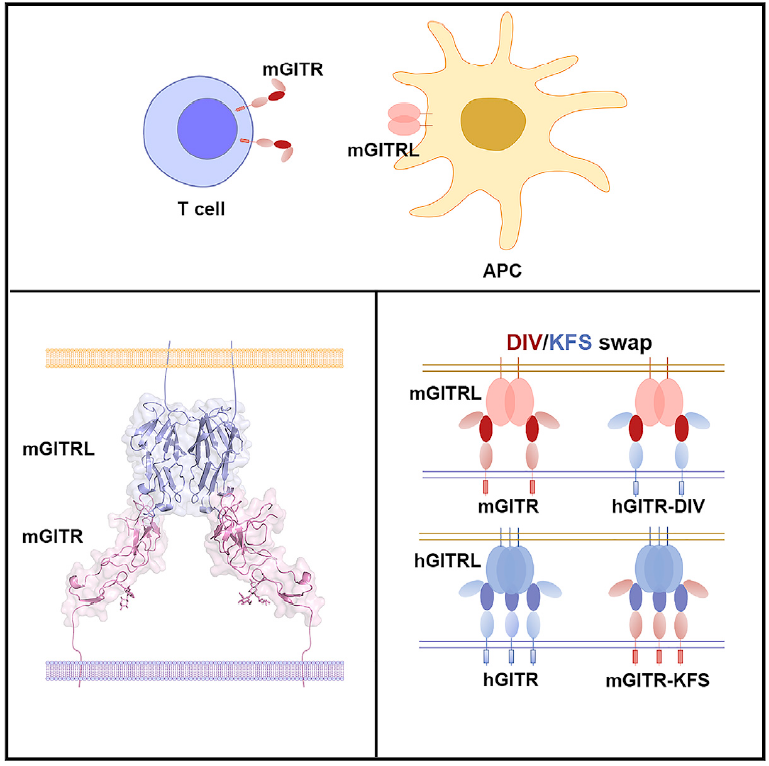
Complex structure of GITR/GITRL and the “DIV/KFS” motif for binding specificity of GITR(Image by Dr. GAO Fu’s Group)
Link of the article: https://doi.org/10.1016/j.celrep.2021.109734