Mycobacterium tuberculosis (Mtb), the causative agent of tuberculosis (TB), poses a continuing threat to patients due to the emergence and spread of antimicrobial resistance. Host immune cells are equipped with cytosolic sensors to detect invading pathogens and initiate anti-infectious responses, but how pathogens such as Mtb undermine host intracellular surveillance for persistent infection remains incompletely understood.
Recently, Prof. LIU Cui Hua’s group at the Institute of Microbiology of Chinese Academy of Sciences (IMCAS) revealed a pathogenic mechanism by which Mtb hijacks the host linear ubiquitin machinery to drive inflammasome sensor NLRP3 degradation, thereby counteracting host intracellular immune surveillance (Figure 1). This work was published in Cell Reports.
The researchers identified that PknG, a Mtb-secreted protein kinase, subverts NLRP3-mediated immune responses by disrupting linear ubiquitin chain assembly complex (LUBAC). Mechanistically, PknG phosphorylates the HOIL-1L subunit of LUBAC, preventing HOIL-1L from participating in LUBAC formation. This disruption impairs the LUBAC-dependent linear ubiquitination of the inflammasome adaptor ASC, ultimately inhibiting NLRP3 inflammasome assembly. Moreover, PknG-mediated phosphorylation concurrently activates HOIL-1L’s intrinsic E3 ubiquitin ligase activity, enabling HOIL-1L to mediate K48-linked ubiquitination and subsequent degradation of NLRP3. To sum up, through this dual-regulation mechanism that achieves the "one-stone-two-birds" effect as mentioned above, PknG can help promote the escape of Mtb from NLRP3-mediated cytoplasmic immune surveillance, thereby further facilitating the intracellular survival and infection of the pathogen.
This study reveals that Mtb exploits the phosphorylation-dependent dynamics of the host linear ubiquitin machinery through the PknG/HOIL-1L interaction. This interaction forms an inter-species enzymatic cascade that drives inflammasome sensor degradation, allowing Mtb to counteract NLRP3-dependent immune surveillance. Importantly, these findings also provide a potential strategy for anti-TB therapy and the optimization of the Bacille Calmette-Guérin (BCG) vaccine by targeting the PknG/HOIL-1L interface.
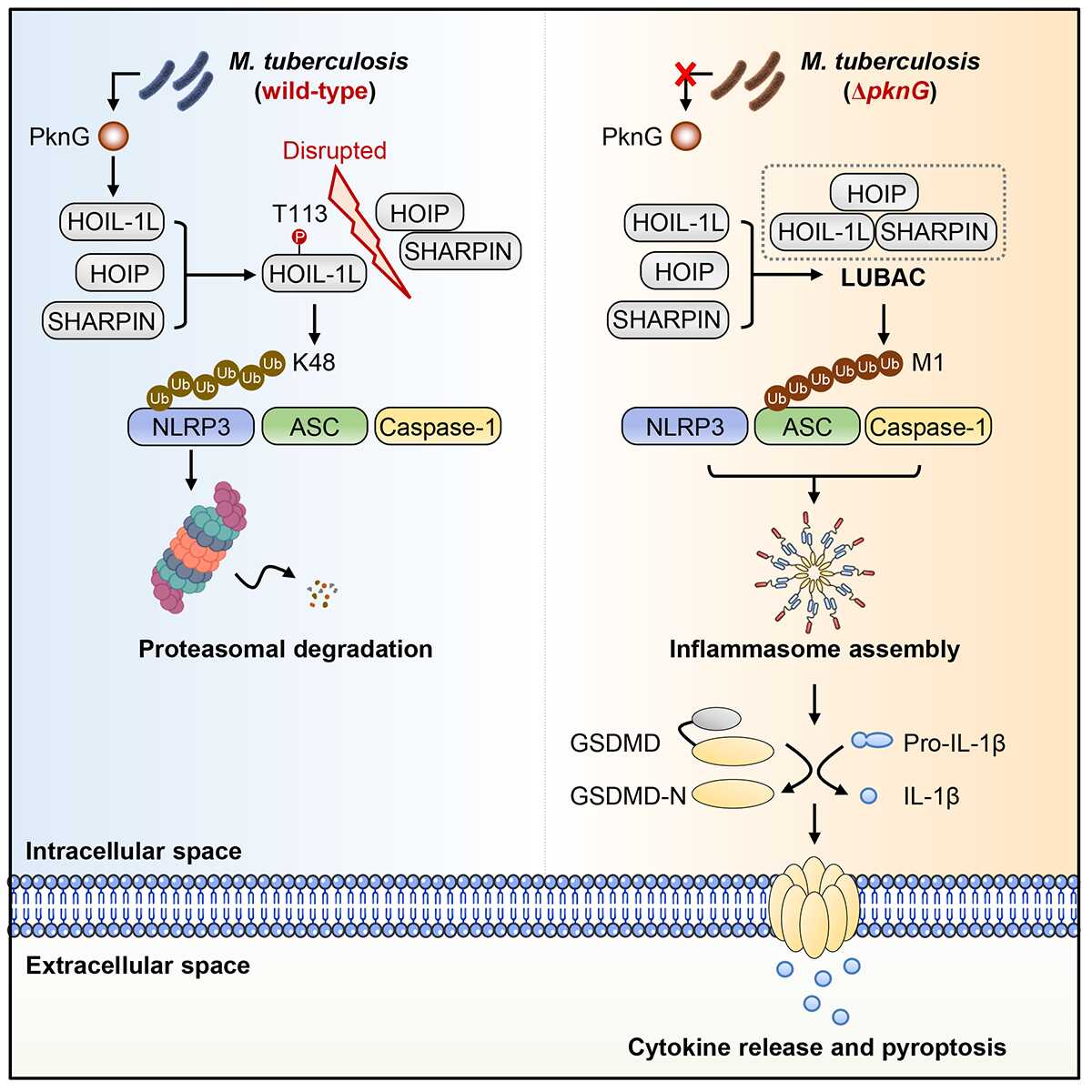
Figure 1. Mtb PknG hijacks the host linear ubiquitin chain assembly complex (LUBAC) to evade NLRP3 inflammasome-mediated cytosolic immune surveillance (Image by Prof. LIU Cui Hua’s group )
Full text links: Pathogenic phosphorylation of linear ubiquitin machinery causes inflammasome sensor degradation