The adhesion mechanism between pathogenic bacteria and host cells represents a fundamental aspect of pathogenic biology. Prof. George F. Gao’ lab from the Institute of Microbiology of Chinese Academy of Sciences identified the key mechanism through which the adhesin CbpF from Fusobacterium nucleatum binds to host receptors CEACAM1 and CEACAM5. This work was published in the Proceedings of the National Academy of Sciences (PNAS).
Pathogenic bacteria employ specialized surface molecules called adhesins to establish tight attachment to host cells via precise molecular interactions. This adhesion process is dynamically regulated during infection, allowing bacteria to adapt to changing conditions. However, how bacteria control the strength of adhesion at the bacterium-host interface has remained poorly understood.
Fusobacterium nucleatum is an anaerobic bacterium notably abundant in the gut microbiota of colorectal cancer patients. It utilizes its adhesin protein CbpF to specifically bind to human cell receptors CEACAM1 and CEACAM5. These receptors are frequently overexpressed on many types of cancer cells. Notably, CEACAM1 also acts as an inhibitory receptor on immune cells — when engaged, it suppresses immune activity. Therefore, elucidating how CbpF interacts with CEACAM1 and CEACAM5 is critical for designing targeted therapeutic strategies that can disrupt bacteria-driven tumor progression.
Using cryo-electron microscopy, the research team resolved high-resolution structures of CbpF bound to CEACAM1 and CEACAM5. In the complex structures, CbpF assembles into a trimer, with each monomer binding one CEACAM1 or CEACAM5 molecule, forming a symmetric 3:3 binding pattern. Additionally, the researchers observed another type of complex in which two CbpF trimers bind to singel receptor dimer.
Based on this findings, the team proposed a “Velcro model” for bacterial adhesion: the highly flexible CbpF protein acts as the “loop” side, interacting with multiple binding sites on the host receptor, which serves as the “hook” side. This mechanism allows bacteria to dynamically adjust adhesion strength under mechanical stress, enabling tight attachment when needed and easy detachment when necessary to adapt to complex physiological environments. This finding reveals how bacteria dynamically regulate adhesion at the molecular level. This work not only enhances the understanding of how Fusobacterium nucleatum colonizes tumor tissues and suppresses immune mechanisms, but also provides key targets for developing novel anti-tumor and antibacterial drugs.
The study was conducted in collaboration with researchers from Renji Hospital, School of Medicine, Shanghai Jiao Tong University, and Institute of Process Engineering, Chinese Academy of Sciences.
This work was supported by the National Key Research and Development Program of China.
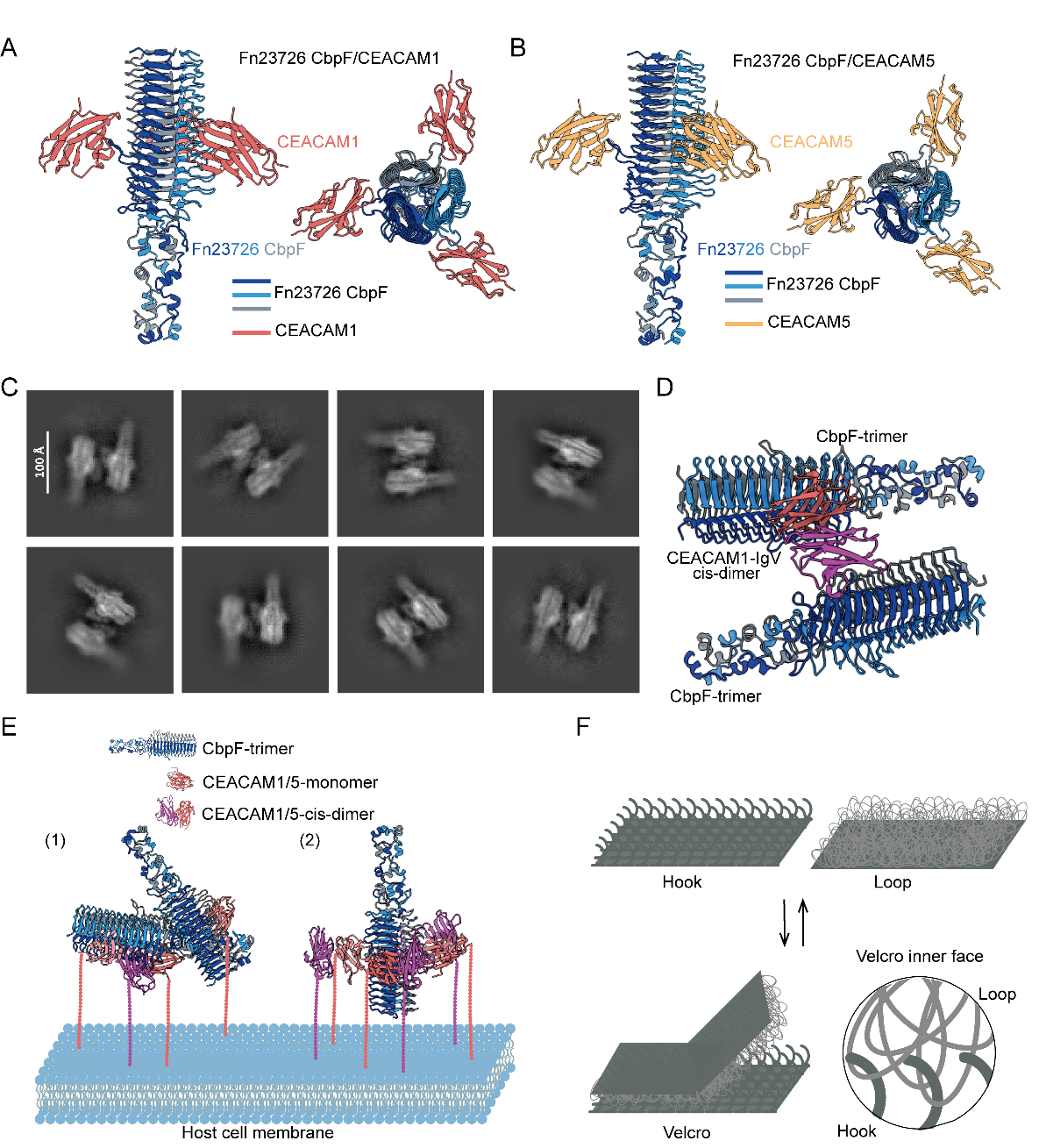
Figure: Structural model of CbpF binding to CEACAM1/CEACAM5 and the proposed "Velcro" adhesion mechanism. (Image by Prof. George F. Gao's group)
Full text link: Binding ofFusobacterium nucleatumautotransporter adhesin CbpF to human CEACAM1 and CEACAM5: A Velcro model for bacterium adhesion