Persistent infection of Mycobacterium tuberculosis (Mtb) has been linked to systemic exhaustion of the host immune system, which exacerbates the progression of tuberculosis (TB). This chronic infectious disease remains a major publichealth threat worldwide. However, the immune checkpoint driving functional exhaustion of TB and the underlying mechanisms remain largely unclear. Recently, Prof. LIU Cui Hua's group at the Institute of Microbiology of the Chinese Academy of Sciences (IMCAS), in collaborating with Prof. WANG Jing and Prof. George Fu Gao from IMCAS as well as Prof. PANG Yu from Beijing Chest Hospital, has identified an immune checkpoint axis constituted by leukocyte immunoglobulin-like receptor B1 (LILRB1) and its ligand human leukocyte antigen-G (HLA-G) that drives natural killer (NK) cell exhaustion in TB. This work was published in EMBO Molecular Medicine.
In this study, researchers combined transcriptional profiling with time-of-flight mass cytometry (CyTOF) to systematically analyze the functional characteristics and immune receptor repertoire of natural killer (NK) cells in individuals at different statuses of Mtb infection. By conducting more in-depth investigations, the researchers further identified LILRB1 as a critical checkpoint receptor that defines a TB-associated NK cell subset and drives NK cell exhaustion in TB patients.
Mechanistically, Mtb-infected macrophages display high expression of human leukocyte antigen-G (HLA-G), which upregulates and activates LILRB1 on NK cells to impair their functions by inhibiting mitogen-activated protein kinase (MAPK) signaling via tyrosine phosphatases SHP1/2. Furthermore, LILRB1 blockade restores NK cell-dependent anti-Mtb immunity in immuno-humanized mice.
This study demonstrates that NK cell checkpoint blockade is a rational strategy to unleash host anti-TB immunity. Moreover, the discovery of the LILRB1–HLA-G axis, which drives the exhaustion of TB-associated NK cells, provides a promising target for TB immunotherapy (Figure 1).
This work was supported by the National Key Research and Development Program of China and the National Natural Science Foundation of China.
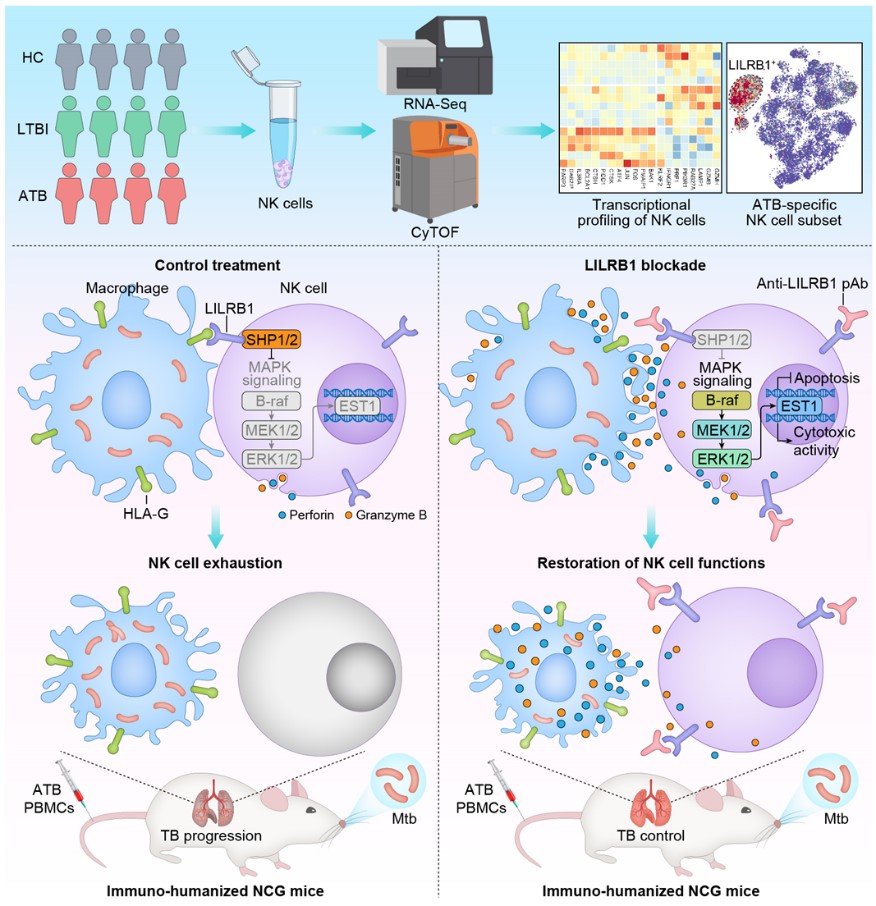
Figure 1. Schematic model showing the mechanism by which LILRB1 blockade restores functions of exhausted NK cells from ATB patients to enhance host anti-Mtb protective immune responses (Image by Prof. LIU’s group)
Contact:
Dr. LIU Cui Hua
Phone: 86-010-64806197
E-mail: liucuihua@im.ac.cn
LILRB1-HLA-G axis defines a checkpoint driving natural killer cell exhaustion in tuberculosis