Tuberculosis (TB) is a major chronic infectious disease caused by M. tuberculosis (Mtb). According to the data provided by the World Health Organization, there were nearly 10.60 million new cases of TB worldwide in 2022 and 1.60 million deaths from Mtb infection. Dr. Cui Hua Liu's group (from the Institute of Microbiology, Chinese Academy of Sciences) has been investigating on the molecular mechanisms underlying pathogen infection and immune regulation, and her group has published a series of articles in top-ranked journals including Science, Nature Immunology, Nature Communications, Proc Natl Acad Sci,Autophagy, Cellular & Molecular Immunology, and EMBO reports. These studies reveal the dynamic processes and molecular mechanisms of Mtb-host interaction, providing new strategies and specific targets for the anti-TB therapy.
Cell death modalities are common outcomes during Mtb infection, among which ferroptosis is a form of cell death induced by iron ion and lipid peroxidation. The host cells infected with Mtb usually exhibit ferroptosis phenotypes including reduced glutathione peroxidase 4 (GPX4) level, increased free iron, mitochondrial superoxide, and lipid peroxidation, which ultimately promote the replication and dissemination of Mtb. But up to now, the specific effector and the underlying molecular mechanisms of Mtb effectors involved in host ferroptosis modulation remain largely unexplored, and these knowledge could provide the theoretical basis and potential targets for TB treatments based on pathogenicity and dissemination of the pathogen.
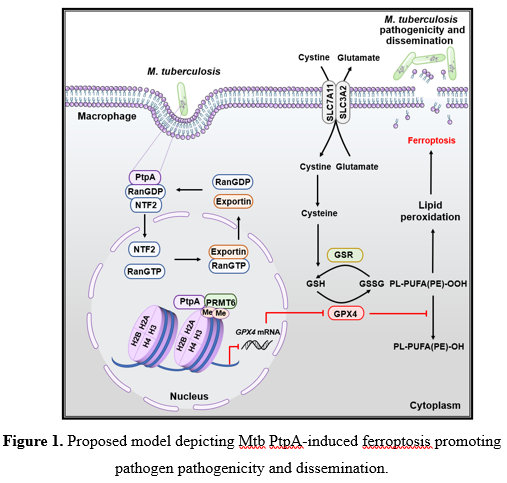
Recently, Jing Wang's group and Cui Hua Liu's group in collaborating with Lingqiang Zhang's group (State Key Laboratory of Proteomics, National Center for Protein Sciences (Beijing), Beijing Institute of Lifeomics) have revealed that Mtb PtpA induces host cell ferroptosis through targeting epigenetic modification, thus enhancing the pathogenicity and dissemination of Mtb (Figure 1). Specifically, in vitro screening experiments and transcriptomic analysis identified Mtb PtpA as an important effector protein involved in host cell ferroptosis induction. Further investigation revealed that Mtb PtpA is located in both cytoplasm and nucleus of host cell during mycobacterial infection. It is worth mentioning that PtpA does not contain currently identifiable nuclear import motif, but enters the nucleus through the interaction between its Cys site and the nuclear entry transporter Ran/NTF2 complex of the host cell. Subsequently, nuclear PtpA binds to protein arginine methyltransferase 6 (PRMT6) via its 1-50 amino acids region to increase asymmetric dimethylation of histone H3 at arginine 2 (H3R2me2a), thus inhibiting GPX4 transcription and expression. Consistantly, in vivo experiments further confirmed the important regulatory role of PtpA-induced ferroptosis in mediating host tissue damage and pathogen dissemination.
The followings highlight the significance and breakthroughs of this study: 1) Identifies an important effector protein inducing ferroptosis during Mtb infection, and reveals the molecular mechanism of Mtb regulating cell death through epigenetic modification. 2) Reveals a non-classical nuclear entry mechanism and the involved key site of Mtb PtpA. 3) Provides a new TB treatment strategy via targeting Mtb PtpA-host PRMT6 interface to inhibit Mtb-induced ferroptosis, thus reducing Mtb pathogenicity and dissemination.
The paper entitled “A mycobacterial effector promotes ferroptosis-dependent pathogenicity and dissemination” has been published online in Nature Communications with Dr. Lihua Qiang, Yong Zhang, and Zehui Lei as joint first authors, Dr. Jing Wang, Dr. Cui Hua Liu, and Dr. Lingqiang Zhang as joint corresponding authors. This work is jointly supported by the National Key Research and Development Program of China, the National Natural Science Foundation of China, the Strategic Priority Research Program of the Chinese Academy of Sciences, Youth Innovation Promotion Association CAS, and the CAS Project for Young Scientists in Basic Research.
Contact:
Dr. Jing Wang
E-mail: wangj6@im.ac.cn
CAS Key Laboratory of Pathogenic Microbiology and Immunology, Institute of Microbiology, Chinese Academy of Sciences, 100101, Beijing, China
Full text link: https://www.nature.com/articles/s41467-023-37148-x