Dr. Cui Hua Liu's group (from the Institute of Microbiology, Chinese Academy of Sciences) has been investigating the molecular mechanisms underlying pathogen infection and immune regulation. In recent years, Liu's group has published a series of papers in top-ranked journals including Science、Nature Immunology、Nature Communications、Proc Natl Acad Sci、Autophagy、Cellular & Molecular Immunology、EMBO reports. These studies reveal the underlying molecular mechanisms involved in pathogen infection and host immune regulation, which providing new strategies and potential targets for the development of treatments against tuberculosis and related inflammatory diseases.
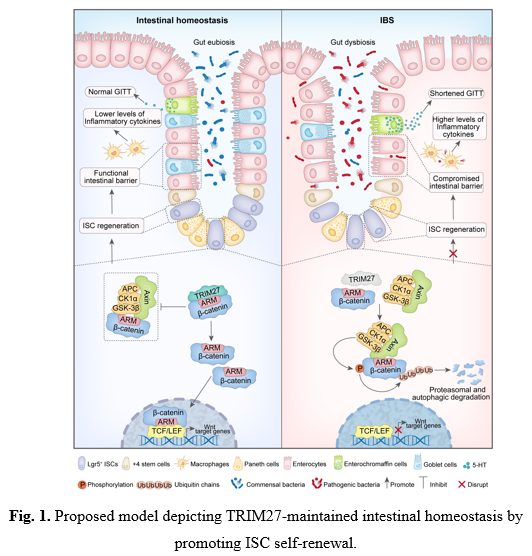
Recently, Dr. Cui Hua Liu’s group, in collaborating with Dr. Jing Wang’s group (also from the Institute of Microbiology, Chinese Academy of Sciences) as well as Dr. Lingqiang Zhang’s group (from National Center for Protein Sciences, Beijing), have revealed that TRIM27 (Tripartite motif containing 27), an E3 ligase of TRIM family proteins, is a critical genetic factor for maintaining intestinal homeostasis. Specifically, through analyzing the expression levels of TRIM family members in intestinal tissues of two most common gastrointestinal disorders sharing overlapping symptoms includingirritable bowel syndrome (IBS) and inflammatory bowel disease (IBD), the E3 ubiquitin ligase TRIM27 was identified to be highly expressed in IBD patients while lowly expressed in IBS patients as compared with that in healthy control group, suggesting that TRIM27 may play a delicate regulatory role in maintaining gut homeostasis. Furthermore, through establishing Trim27-knockout mouse models, they found that Trim27 deficiency induced IBS-like symptoms in mice, including low-grade intestinal mucosal inflammation, intestinal barrier impairment, visceral hypersensitivity, altered gastrointestinal motility and gut dysbiosis, which phenomenon gradually aggravates with the increasing of mouse age. Mechanistically, TRIM27 bounds to the conserved Armadillo (ARM) repeat of β-catenin in its Coiled-coil (CC) domain-dependent manner, independently of its E3 ubiquitin ligase activity, followed by preventing β-catenin from binding to Axin to inhibit the degradation of β-catenin, resulting in the activation of Wnt/β-catenin signaling to promote ISC self-renewal. Furthermore, Wnt/β-catenin signaling activator SKL2001 and probiotics treatment alleviates IBS-like symptoms in Trim27-knockout mice through promoting ISC self-renewal (Figure 1).
The followings highlight the significance and breakthroughs of this study:1)This study reveals TRIM27 asa critical genetic protective factor for IBS.Importantly,Trim27-knockout mouse models established in this study spontaneously develops a series of symptoms similar to those of IBS-D patients, and could be serve as a stable genetic-based tool for further mechanistic studies and drug development for IBS. 2) This study elucidates the regulatory mechanism of ISC homeostasis and its mediated intestinal epithelial barrier integrity in the pathogenesis of IBS. 3) This study provides a potential treatment for IBS via targeting the TRIM27/Wnt/β-catenin axis, and suggests that TRIM27 might serve as a biomarker for differentiating IBS from IBD.
The paper entitled “TRIM27 maintains gut homeostasis by promoting intestinal stem cell self-renewal” has been published online in Cellular & Molecular Immunology with Dr. Jing Wang, Dongdong Zhao, Zehui Lei and Pupu Ge as joint first authors, Dr. Cui Hua Liu and Dr. Lingqiang Zhang as joint corresponding authors. This work is jointly supported by the National Key Research and Development Project of China, the National Natural Science Foundation of China, the Strategic Priority Research Program of the Chinese Academy of Sciences, Youth Innovation Promotion Association CAS and the State Key Laboratory of Proteomics.
Contact:
Dr. Cui Hua Liu
E-mail: liucuihua@im.ac.cn
CAS Key Laboratory of Pathogenic Microbiology and Immunology, Institute of Microbiology, Chinese Academy of Sciences, 100101, Beijing, China
Full text link: https://www.nature.com/articles/s41423-022-00963-1