Tuberculosis (TB) is a chronic infectious disease caused by M. tuberculosis (Mtb) and remains a serious threat to human health. According to the report from the World Health Organization (WHO), about 10.00 million people were estimated to have fallen ill with TB and 1.41 million people died from TB in 2019 (WHO, 2020). Mtb is a successful intracellular pathogen that employs multiple strategies to subvert host cellular functions for immune evasion, ultimately promoting their prolonged intracellular survival. Dr. Cui Hua Liu's group (from the Institute of Microbiology, Chinese Academy of Sciences) has been investigating the molecular mechanisms underlying Mtb-host interactions, especially focusing on how Mtb hijacks the ubiquitin (Ub) system to escape host immune clearance and how host Ub system promotes anti-infection innate immunity. In recent years, Liu's group has published a series of papers in top-ranked journals including Nature Immunology, Nature Communication, Proc Natl Acad Sci,Cellular & Molecular Immunology. These studies reveal the dynamic processes and the underlying molecular mechanisms of Mtb-host interactions, and provide new strategies and specific targets for anti-TB theraputics.
Mtb secretes a series of eukaryotic-type kinases and phosphatases that are speculated to mediate the cross-talk between Mtb and host immune signaling pathways. Among these eukaryotic-type kinases and phosphatases, the mycobacterial phosphatase PtpA and kinase PknG are of particular interest due to their critical role in Mtb intracellular survival and pathogenicity, and are thus deemed as prime targets for the development of novel TB therapeutics. However, the specific mechanisms underlying Mtb PtpA/PknG-mediated regulation of host immune functions, including related cellular biological processes, key signaling pathways and targets, as well as the involved protein-protein interactions and biochemical mechanisms, have not been fully elucidated. Therefore, better understanding of the molecular mechanisms underlying Mtb PtpA/PknG-host interactions will be invaluable in developing novel effective and selective TB therapies based on specific Mtb-host interfaces. Previous studies from Dr. Cui Hua Liu's group reveal that the cytoplasmic Mtb PtpA can hijack host Ub and then is activated to dephosphorylate phosphorylated kinase JNK and p38, thus leading to the suppression of innate immunity (Nature Immunology, 2015), while the nuclear PtpA binds to the promoter of GADD45A gene to promote tumor cells proliferation and migration (Nature Communications, 2017).
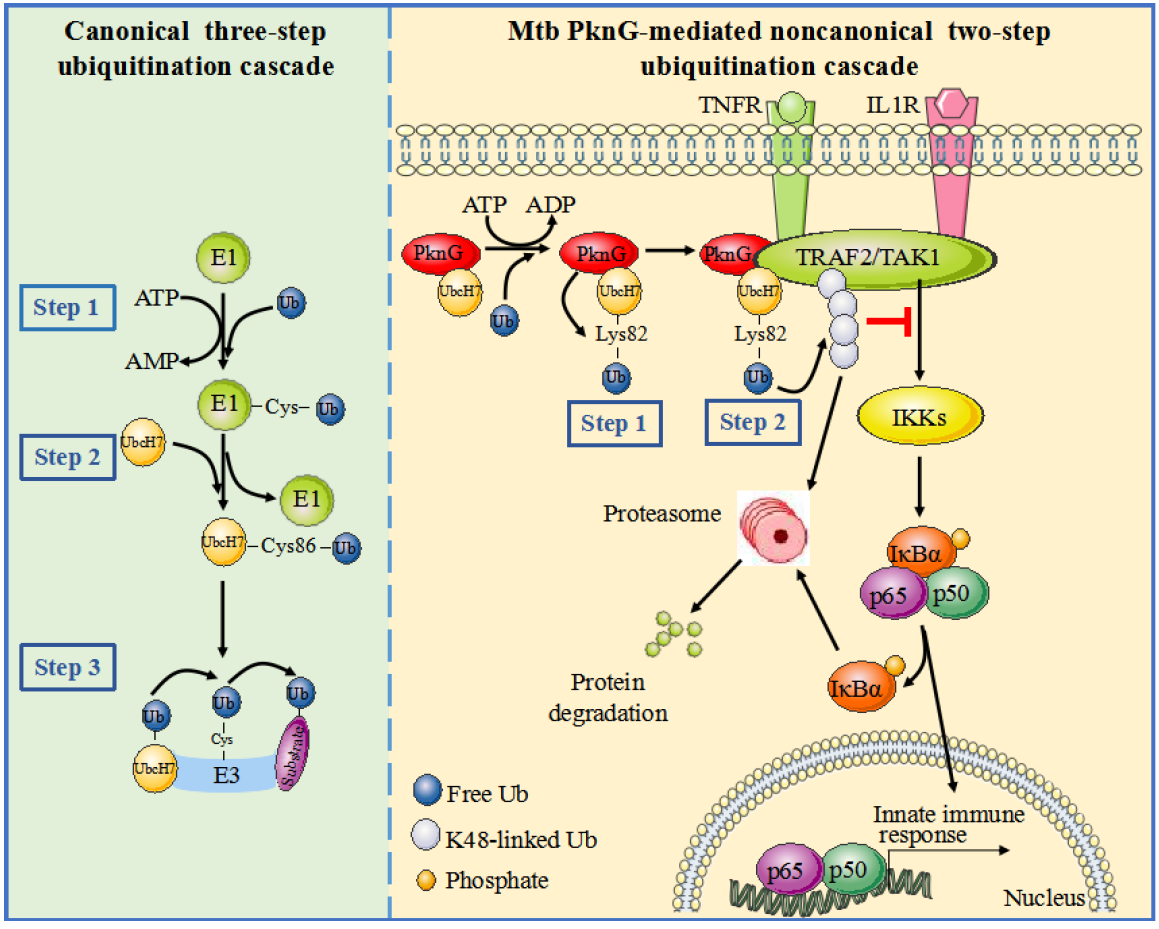
Recently, Dr. Cui Hua Liu’s group, in collaborating with Dr. Xiao-Bo Qiu (from Beijing Normal University) and Dr. George Fu Gao (from the Institute of Microbiology, Chinese Academy of Sciences), has revealed that Mtb PknG directly interacts with another important component of the host Ub system, the Ub conjugation enzyme (E2) UbcH7, to attack host innate immunity through catalyzing a novel two-step ubiquitination cascade.
The followings highlight the significance and breakthroughs of this study:
1) Mtb PknG serves as both the ubiquitin-activating enzyme (E1) and the ubiquitin ligase (E3) to promote the ubiquitination and degradation of host tumor necrosis factor receptor-associated factor 2 (TRAF2) and TGF-β-activated kinase 1 (TAK1), and thus inhibits NF-κB signaling activation. To the best of our knowledge, Mtb PknG is the first mycobacterial effector protein to be identified as a dual functional E1 and E3 enzyme targeting host protein substrates.
2) The canonical ubiquitination of target proteins involves a three-step enzymatic process that is typically catalyzed by E1, E2 and E3 sequentially. Instead, PknG activates Ub and then promotes the ubiquitination of the substrate through two sequential steps. In the first step, PknG interacts with UbcH7 via a novel ubiquitin-like (Ubl) domain and acts as an unconventional E1 to catalyze the covalent conjugation of Ub with UbcH7, and then functions as an isopeptidase to release Ub from UbcH7-Ub conjugates to obtain the activated Ub. Surprisingly, PknG directly catalyzes Ub conjugation onto Lys82 of UbcH7 via an isopeptide bond, bypassing the formation of an intermediate thioester bond with Ub, while Ub is usually transferred to Cys86 of UbcH7 by the classical E1 to form a thioester bond, accompanied by the formation of an E1-Ub thioester linkage. In the second step, the released Ub is transferred to the substrate by PknG through its E3 activity.
3) More strikingly, Mtb PknG uses ATP to catalyze E2-Ub conjugation via an isopeptide bond with the release of ADP, a phenomenon different from that of the conventional E1s that usually use ATP to facilitate the conjugation of E2-Ub with the release of AMP. The findings from this study update our understanding of the catalytic mechanisms involved in ubiquitination process, and provide important information for rational development of TB treatment via targeting the unconventional E1 and E3 activities-associated Ubl domain of PknG, which exhibits no homology to human hosts (Figure 1).
The paper entitled “M. tuberculosis protein kinase G impairs host immunity by acting as an unusual ubiquitinating enzyme” has been published online in EMBO reports with Dr. Jing Wang, Pupu Ge and Zehui Lei as joint first authors and Dr. Cui Hua Liu, Dr. Xiao-bo Qiu and Dr. George Fu Gao as joint corresponding authors. This work was supported by grants from the National Natural Science Foundation of China, the Strategic Priority Research Program of the Chinese Academy of Sciences, the National Key Research and Development Program of China, the National Science and Technology Major Project, and the Youth Innovation Promotion Association CAS.
Access to this published article via this link: http://doi.org/10.15252/embr.202052175