Recently, George F. Gao and Feng Gao from Chinese Academy of Sciences (CAS) in Beijing and their colleagues, reveal how Chikungunya virus binds its receptor on human cells by structural biology and virology methods. The article entitled “Molecular Basis of Arthritogenic Alphavirus Receptor MXRA8 Binding to Chikungunya Virus Envelope Protein” was published in Cell On May 9, 2019 (https://www.cell.com/cell/fulltext/S0092-8674(19)30394-0).
Arthritogenic alphaviruses, such as Chikungunya virus (CHIKV), Ross River virus (RRV), Mayaro virus (MAYV), and O’nyong-nyong virus (ONNV), cause severe and debilitating rheumatic diseases worldwide, resulting in severe morbidity and economic costs. One of these, CHIKV, is a serious disease in many tropical and subtropical countries throughout the world. The CHIKV infection is characterized by acute and chronic symmetrical peripheral polyarthralgia-polyarthritis, and severe diseases, even fatalities, have been reported in recent outbreaks. However, no licensed vaccine or antiviral therapy is available.
Alphaviral invasions of susceptible cells are mediated by the envelope (E) glycoproteins, which form icosahedral shells at the virion surfaces. Like other alphaviruses, CHIKV entry is mediated by two glycoproteins, E1 and E2, on the surface of the virion. It is believed that the glycoprotein E2 is responsible for receptor binding, while E1 is responsible for membrane fusion. A recent study showed that multiple emerging arthritogenic alphaviruses, including CHIKV, RRV, MAYV, and ONNV, use matrix remodeling-associated protein 8 (MXRA8) as a functional receptor. How this receptor binds the envelope protein is the key question to be addressed in this study, which will be helpful for the development of countermeasures against these viruses and the understanding of the viral entry into the cells.
The team solved the crystal structures of the mouse MXRA8, human MXRA8 in complex with the CHIKV E protein, and the cryo-electron microscopy structure of human MXRA8 and CHIKV virus-like particle, and they found that MXRA8 has two Ig-like domains that display unique structural topologies, different from those of the previously described two-domain Ig-like molecules. Domain 1 (D1) is formed by two discrete fragments, while the region between these two fragments consists of the hinge region and domain 2 (D2); that is, the linear protein sequence crosses the two domains. Therefore, the two Ig-like domains are connected by two hinge loops. The binding mode of MXRA8 to CHIKV E is also unique. MXRA8 binds in the “canyon” between two protomers of the E spike on the virion surface, with the involvement of both the E1 and the E2 proteins. The atomic details of the interface between the two binding entities reveal that the two domains and the MXRA8 hinge region all interact with the CHIKV E1-E2 residues from two protomers. The critical interactions observed in the complex structure were further demonstrated by site-directed alanine-scanning mutagenesis and surface plasmon resonance (SPR) experiments. In addition, they showed that the stalk region of MXRA8 was necessary for efficient binding and entry.
The identification of multiple binding interfaces for receptor-envelope interaction might inform the development of novel vaccines and broadly neutralizing antibodies. Their findings will help to drive the development of powerful antiviral reagents against arthritogenic alphaviruses.
Hao Song, an assistant professor from Beijing Institutes of Life Science CAS, Ms Zhennan Zhao and Dr. Yan Chai from Institute of Microbiology CAS, are co-first authors of this paper. Prof. George F. Gao and Feng Gao from Tianjin Institute of Industrial Biotechnology CAS are co-corresponding authors of this paper. This work was supported by the National Key Research and Development Program of China, the Strategic Priority Research Program of CAS, the China National Grand S&T Special Project, the National Natural Science Foundation of China, the Youth Innovation Promotion Association CAS and the Young Elite Scientist Sponsorship Program of China Association for Science & Technology (CAST).
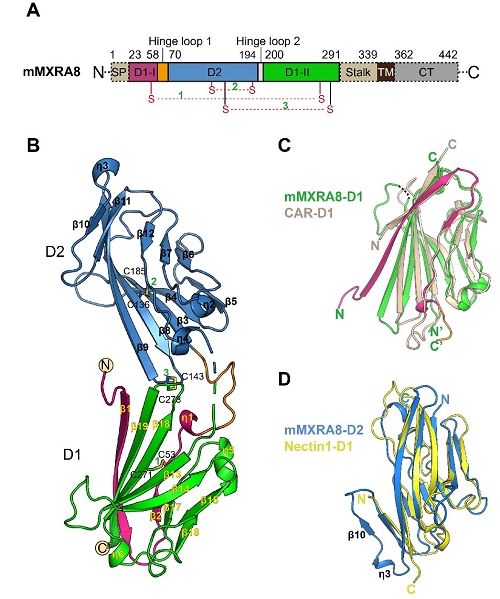
Figure 1. Overall structure of the ectodomain of mouse MXRA8
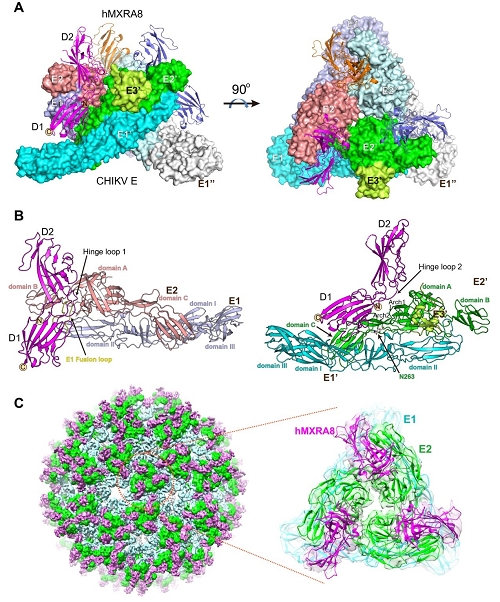
Figure 2. The complex structure of CHIKV E3-E2-E1 glycoprotein bound to human MXRA8
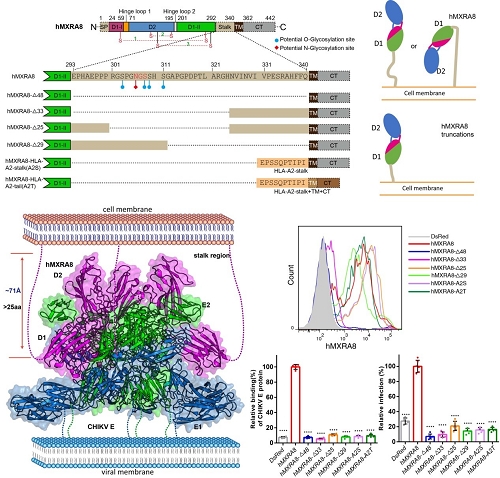
Figure 3. The stalk region of MXRA8 is critical both for cell surface expression and for CHIKV entry
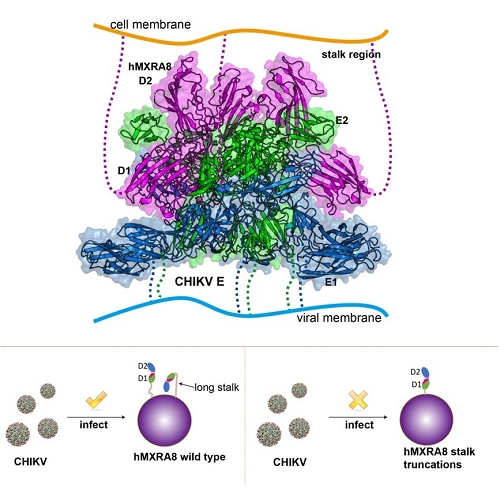
Figure 4. The mechanism of CHIKV entry mediated by MXRA8 receptor