Gp96, an abundant heat shock protein residing within ER, plays a critical role in protein folding, degradation, cross-presentation of viral and tumor antigens, and induction of CTL immune response.
With more than 20 years of research work led by Professor MENG Songdong from Institute of Microbiology, Chinese Academy of Sciences, his research team has achieved significant outcome concerning gp96’s function under both normal and pathological conditions.
Relative research work has been published on Lancet, Molecular Oncology, and Cancer Letters, etc. Prof. MENG has been authorized with ten patents, including one by American authority and nine by Chinese authority. Two candidate drugs based on his research have been under safety evaluation and manufacturing development.
Currently, MENG’s team focuses on gp96’s peptide-binding properties and associated peptides of gp96 purified from various tissues. Through mass spectrum analysis, new sequences of peptides associated with gp96 purified from HBV-transformed hepatocellular carcinoma or human placenta were identified.
They demonstrated that a seasonal influenza vaccine adjuvanted by gp96 elicits cross-protection against different influenza infection.
MENG also discovered that in most cancer cells gp96 translocates from ER to the cell membrane, forming heteromultimer with major tumor proteins such as HER2, uPAR, and ER-α36.
In addition, he discovered that high-dose gp96 immunization, in contrast to its conceived function of CTL activation under normal dose injection, specifically activates Treg through its interaction with Treg’s membrane TLR-2 and TLR-4 molecules. By suppressing humoral and cell immune responses, high-dose gp96 immunization has the potential to treat various autoimmune diseases.
By revealing the peptide-binding properties and immunological amplification mechanism of gp96 molecule, MENG’s work proved the possible design of a universal influenza vaccine and other new types of HBV and cancer vaccines.
The critical role of membrane gp96 in stabilizing membrane HER2, uPAR and ER-α36 suggests that gp96 is an effective target protein for cancer drug development.
Besides, the activation of Tregs and subsequent induction of immunosuppression process by high-dose gp96 immunization also shed light into the development of new therapies for autoimmune diseases such as SLE, type Ⅰ diabetes and ankylosing spondylitis.
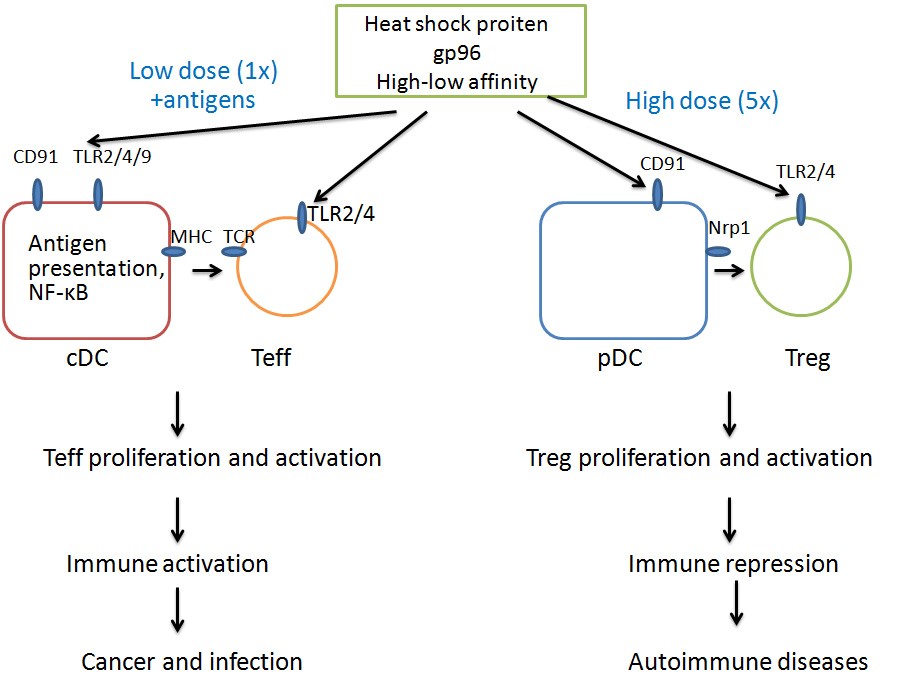
Dose dependent induction of immune activation or repression by gp96 (Image from MENG’s lab).
Contact:
Prof. MENG Songdong
CAS Key Laboratory of Pathogenic Microbiology and Immunology,Institute Of Microbiology,Chinese Academy of Sciences,100101,Beijing, China
E-mail: mengsd@im.ac.cn