Diverse saccharide moieties of microbial natural products influence the pharmacological properties of these compounds dramatically. In microbial natural products, heptoses can be classified into four groups structurally: heptofuranoses, highly reduced heptopyranoses, L-heptopyranoses and D-heptopyranoses. The biosynthetic mechanisms of heptoses belonging to the first two groups have been well elucidated, while, very little is known about the biosynthesis of L-heptopyranoses and D-heptopyranoses, represented by septacidin and hygromycin B respectively. A paper published on Proc Natl Acad Sci USA by Yihua Chen’s Lab in Institute of Microbiology, CAS has made progresses on the biosynthesis of those heptoses recently.
Heptoses exist in the core region of lipopolysaccharide (LPS) of Gram-negative bacteria cell wall. These heptoses are biosynthesized using sedoheptulose-7-phosphate as a precursor, by isomeration (GmhA), phosphorylation at C1 position (HldE), dephosphorylation at C7 position (GmhB), adenylylation (HldE) and C6 epimeration of the hydroxyl group (HldD) to form an intermediate ADP-L-glycero-β-D-manno-heptose and transferred to the aglycon further (Fig 1).
Septacidin, discovered as an antifungal and antitumor compound from Gram-positive bacteria, is found also to be an immunogenic cell death inducer recently. One of its derivatives, KRN5500, is now an anticancer and pain relief drug under clinical trials. Here Yihua Chen and his team showed that the heptose of septacidin is also derived from D-sedoheptulose-7-phosphate and use the same biosynthetic logic as that in the LPS biosynthesis (Fig 1). Septacidin producer, a Gram-positive bacterium, shares the same ADP-heptose biosynthesis pathway with Gram-negative bacterium lipopolysaccharide biosynthesis. These findings showed that the primary metabolite of Gram-negative bacteria and secondary metabolite of Gram-positive bacteria share a conserved heptose biosynthetic pathway. Worthy of particular note is the involvement of ADP-sugar in microbial natural product biosynthesis, which indicates that, besides GDP-, CDP-, UDP-, and dTDP-sugar, ADP-sugar may also serve as a donor for natural product glycosylation. (Fig 1).
Hygromycin B and its resistance gene are a frequently used screening system in molecular biological research. And it is also an anthelmintic agent practically used in swine and poultry farming. Hygromycin B contains a unique D-heptopyranos, which is also derived from D-sedoheptulose-7-phosphate. It was showed that HygP, together with HldE and GmhB from E. coli, was able to efficiently convert D-sedoheptulose-7-phosphate into ADP-D-glycero-β-D-altro-heptose. These findings set the stage for the generation of novel septacidin derivatives by combinatorial biosynthesis and structural modification of the heptoses in the LPS inner core of gram-negative bacterium. Both avenues are currently being actively pursued in our laboratory.
This study was supported by Ministry of Science and Technology of China, National Natural Science Foundation of China, the Youth Innovation Promotion Association of CAS.
On-line paper: https://doi.org/10.1073/pnas.1711665115
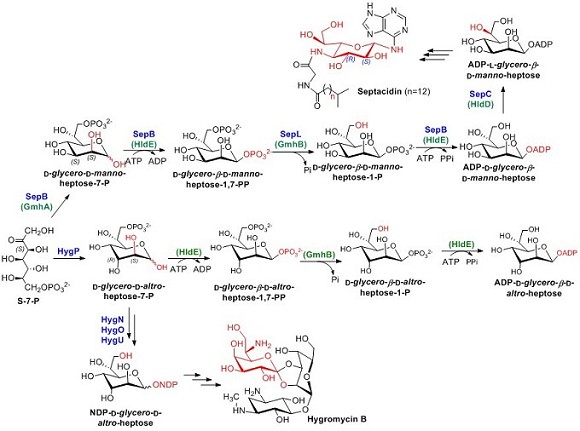
Figure 1. The proposed biosynthetic pathways of heptoses in septacidin, hygromycin B and LPS of E. coli cell wall. The enzymes involved in Gram-negative bacteria LPS heptose biosynthesis are bracketed and indicated in green. Please note that a hybrid reaction of HygP, HldE and GmhB can generate ADP-D-glycero-β-D-altro-heptose.
Contact:
Dr. CHEN Yihua
State Key Laboratory of Microbial Resources, Institute of Microbiology, Chinese Academy of Sciences, No.1 West Beichen Road, Chaoyang District, Beijing 100101
E-mail: chenyh@im.ac.cn